Aluminium Hydride Reagents
Mechanism + Description
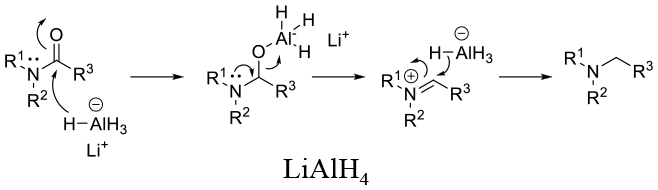
Mechanism involves hydride addition followed by C-O bond cleavage –possibly via an iminium species (or concerted collapse by intramolecular Hydride delivery). A driving force is the Formation of the Al-O bond.

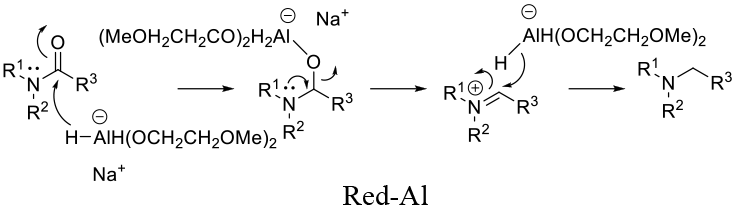
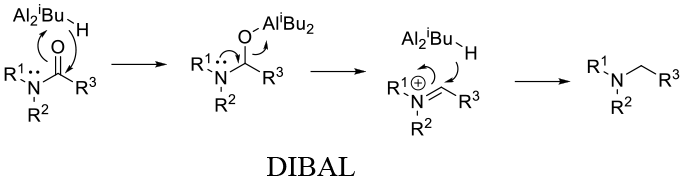
General comments
The three most common Al hydride employed for amide reduction are lithium aluminium hydride (LAH), Sodium bis(2-methoxyethoxy) aluminum hydride (Vitride, Red –Al) and diisobutyl aluminium hydride (DIBAL). Reactivity is LAH > Red –Al > DIBAL. All are powerful reducing agents and compatibility with other reducible functional groups can be problematic. Occasionally some selectivity can be obtained by the correct choice of reagent, solvent and temperature. All reagents generate Al salts on hydrolysis and hydrogen. DIBAL will also generate isobutane.
LAH – Works on 2° and 3° lactams as well as 1° amides. Its used as solid LiAlH4 or a solution of LiAlH4. THF complex in toluene. Solvent: THF /ethers or toluene
Red-Al – Works on 2o (lactam) and 3o amides Solvent: toluene, ethers (10 °C to reflux)
DIBAL – Works on 3° lactams , Solvent: toluene (aliphatic or aromatic hydrocarbons)
Key references
J. Org. Chem., 1953, 18, 1190–1200 The Reduction of Acid Amides with Lithium Aluminum Hydride
e-EROS Encyclopedia of Reagents for Organic Synthesis Sodium Bis(2-methoxyethoxy)aluminum HydrideOrg. Synth. 2006, 83, 141 reduction of pyridinone to piperidine with LiAlH4
Org. Synth. 2006, 83, 70 reduction of aliphatic t-amide with LiAlH4
Relevant scale up example
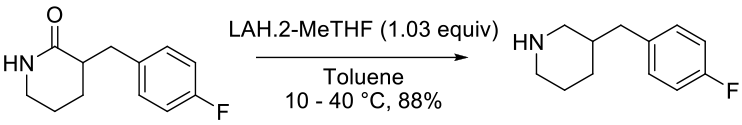
Experimental
7 kg scale
Org. Process Res. Dev. 2006, 10, 262-271
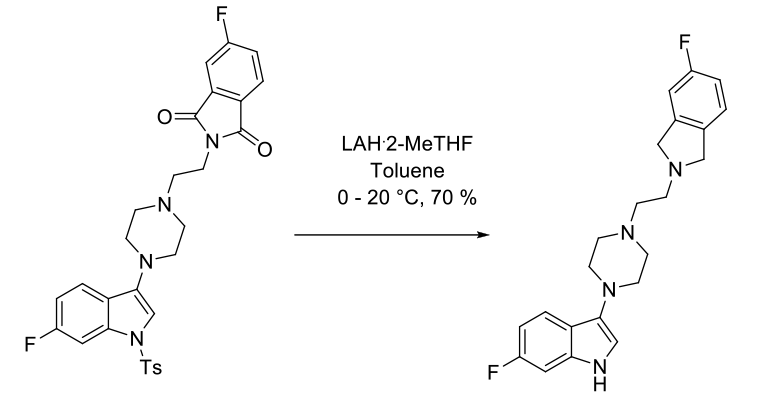
Experimental
6 kg scale
Org. Process Res. Dev. 2003, 7, 521-532

Experimental
2 kg scale
Org. Process Res. Dev. 2013, 17, 61−68
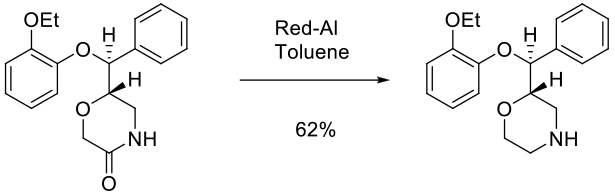
Experimental
100 kg scale
Org. Process Res. Dev. 2007, 11, 346-353.
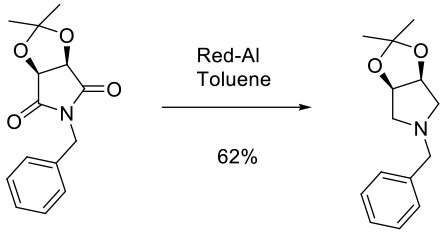
Experimental
150 kg scale
Contains DOE study
Org. Process Res. Dev. 2004, 8, 834-837
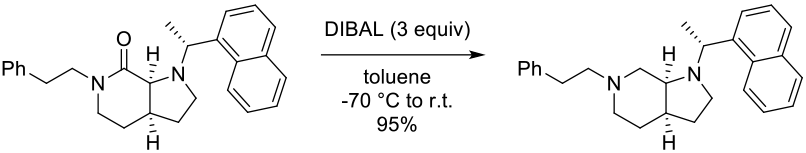
Experimental
40 g scale
Org. Process Res. Dev. 2007, 11, 711-715
Green Review
-
Atom efficiency (by-products Mwt)
- LiAlH4
By-products: 2 eq LiOH (24 g/mol) + 2 eq Al(OH)3 (78 g/mol) + 2 eq H2 gas (2 g/mol) = 208 g by-products/mol amide. Large quench volumes generate further waste - DIBAL-H
By-products: 2 eq Al(iBu)2OH (158 g/mol) = 316 g by-products/mol amide - Red-Al
By-products: 2 eq of NaOH (40 g/mol), 2 eq AlH(OEtOMe)2OH (195 g/mol) = 470 g by-product/mol amide
- LiAlH4
- Safety Concerns
Major concerns are around the pyrophoric nature of most Aluminium hydride reagents, and generation of hydrogen during reaction and quench. Red-Al is the least pyrophoric of this class of reagent. - Toxicity and environmental/aquatic impact
Aluminum hydrides can cause severe burns. In the environment rapid hydrolysis to insoluble Aluminum oxide /hydroxides will occur (pH range 5 to 8). Typically regarded as non-toxic, concern over possible Al toxicity is rising. - Cost, availability & sustainable feedstocks
Most Aluminum hydride reagents are available in bulk at reasonable cost. They are not available from renewable sources. - Sustainable implications
None with Aluminum. Lithium is at moderate risk of depletion. LiAlH4 reactions are being reported in greener ether solvents like CPME and 2-methylTHF