Hydrosilyation
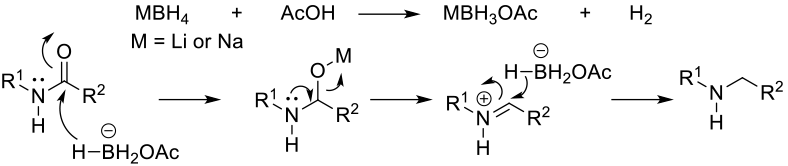
Mechanism + Description
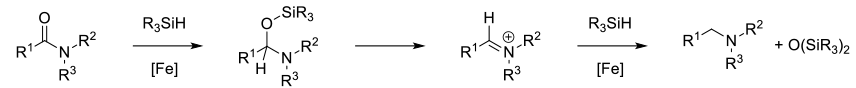
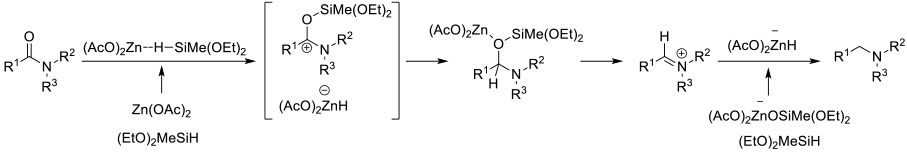
A number of silane reagents (RXSiHY and RXOZSiHY) will reduce amides to amines in the presence of metal catalysts. Typically these reactions proceed through the intimacy of O-silyl hemiaminals. Depending on the catalyst/silane, can get good functional group compatibility.
A related transformation, but mechanistically distinct, is the reduction of primary carboxamides which go via dehydration to the nitrile followed by reduction.
General comments
Amides can be reduced with SiH reagents in the presence of metal catalysts. Traditionally focused on PGM metals like Rh, Ru, Pt, Pd, Ir, Os, Re, base metals like Ti, Fe and Zn can also provide active catalysts. Tert amides are most reactive, but some catalysts will reduce secondary and primary amides. This does allow for some selectivity in molecules with multiple amide groups. A wide range of silanes have been reported for catalysed amide reduction –Et3SiH, (EtO)3SiH, PhSiH3, Me(EtO)2SiH, Polymethylhydrosiloxane (PMHS), Me2PhSiH and (Me2SiH)2O. Solvents are usually ethers or hydrocarbons.
Care needs to be exercised with alkoxysilanes as the hydride donor.
In the presence of Lewis acids or metal catalysts, a metathesis reaction can occur to generate SiH4 which is highly flammable and explosive in the presence of air.
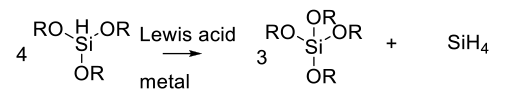
Org. Process Res. Dev. 2010, 14 ,484–484 On the Perils of Unexpected Silane Generation
Key references
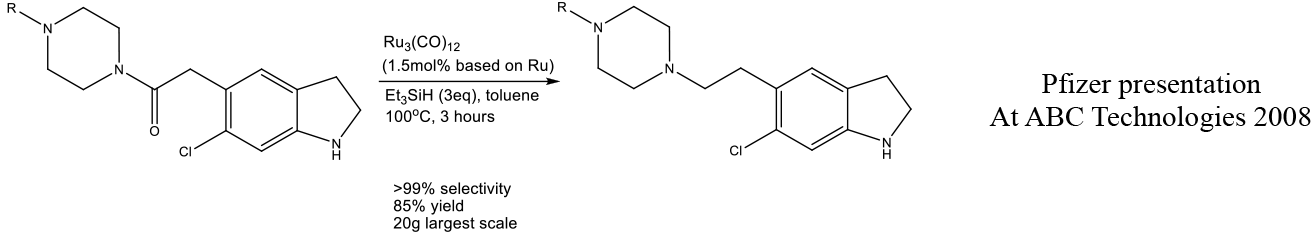
Relevant scale up example