Br2 and Electorphilic Br+ reagents
Mechanism + Description
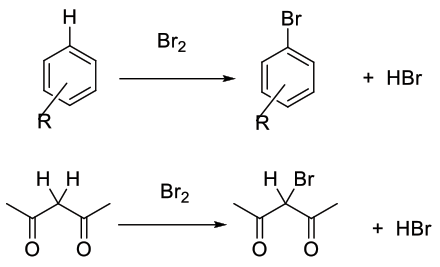
Br2 and Br+ equivalents are electrophilic reagents normally used to add to unsaturated C-C bonds, take part in electrophilic substitution reactions with aromatics/heteroaromatics, or react with acidic C-H bonds. The attacking species is often written as Br2 or Br+, but maybe more complex than these simple species. Br2 and aqueous chlorine can produce HOBr, Br2O, BrCl and BrOCl.
General comments
Br2 is a moderate electrophile, and will react with unsaturated bonds and some (hetero)aromatics, carbanions and enols. It is often activated by strong acids, Lewis acids and oxidants. Oxidants can boost the electrophilic power of the reagent and can also generate Br2 / Br+ in situ from simple bromide salts or HBr. Oxidants can also reoxidise any Br- produced and maximise the use of bromide.
H2O2 or urea H2O2 complex
O2
CAN
Selectfluor
Chloramine-T
m-CPBA
Oxone® (KHSO5 0.5KHSO4 0.5K2SO4)
NaIO4
H5IO6
KClO3
PhI(OAc)2
HOAc
HNO3
Oleum
H2SO4
NH4OAc
Bromination using Br2 or Br2 generated in situ is normally the most atom efficient and sustainable bromination chemistry. Other common reagents can be classified as below :
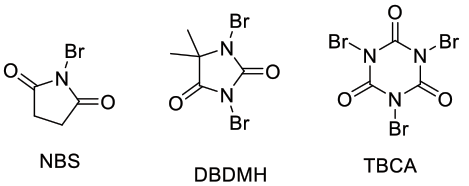
- N-Br compounds
NBS, DBDMH, TBCA – these can act as either sources of electrophilic bromine or radical bromine.
- Br2 complexes – typically Br3 – salts with amine cations. If used as a stoichiometric source of Br+, these salts are quite wasteful in bromine. A combination of NBS and n-Bu4NBr shows similar reactivity to n-Bu4NBr3
- High-valent bromine compounds (such as bromates).
Key references
Tetrahedron Lett. 2012, 53, 191 – Oxidative bromination of ketones using ammonium bromide and oxone
Synlett., 2008, 8, 1267-1268 KX/H2O2 – An Efficient and Non-Polluting Halogenating Reagent
Green Chem., 2007, 9, 1212-1218 – Bromination of ketones with H2O2-HBr in water
Current Green Chem., 2014, 1(2), 94-107 – Tribromoisocyanuric Acid: A Green and Versatile Reagent
Synthesis, 2007, 1471-1474 – A New Chemoselective Synthesis of Brombuterol (PhMe3NBr3)
J. Med. Chem., 2011, 54, 8616–8631 – Identification of Benzoxazin-3-one Derivatives as Novel, Potent, and Selective Nonsteroidal Mineralocorticoid Receptor Antagonists – Example of bromination with pyridinium bromide perbromide
Synthesis, 2006, 0221–0223 – A New RegioselectiveBromination of Activated Aromatic Rings TCBA
J. Org. Chem. 2015, 80, 3701-3707 – Bromination of Olefins with HBr and DMSO
Current Green Chemistry, 2014, 1, 94-107 – Tribromoisocyanuric Acid: A Green and Versatile Reagent
Green Review
-
Atom efficiency (by-products Mwt)
HBr, Na/KBr salts and Br2 with activators/(re)-oxidants are the most atom efficient and maximize the use of bromine. The use of other Br+ reagents are less atom efficient - Safety Concerns
Bromination reactions can be very exothermic especially when activators and oxidants are added. Radical brominations can suffer from delayed exothermic events. Care should be taken with solvent compatibility – (see solvents section). The operational hazards associated with electrophilic and radical bromination can be somewhat mitigated by using flow chemistry. High valent Bromine compounds and N-Br compounds can cause fire, and may be explosive in combination with other materials.
Org. Process Res. Dev., 2013, 17, 145−151 – Application of Flow Photochemical Bromination in the Synthesis of a 5-Bromomethylpyrimidine Precursor of Rosuvastatin: Improvement of Productivity and Product Purity
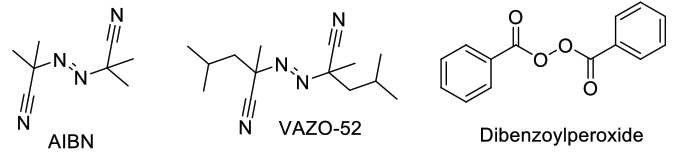
Chemical radical sources used for radical bromination are often radical polymerisation initiators. The hazardous Dibenzoylperoxide has largely been replaced by AIBN, although this initiator can leave toxic residues. VAZO-52 represents a good compromise.
Environ Health Perspect., 1990 (Jul), 87, 309–335 – Toxicity and carcinogenicity of potassium bromate -a new renal carcinogen - Toxicity and environmental/aquatic impact
High concentrations of Br2 and Br+ reagents will be extremely toxic to aquatic organisms though are probably too reactive to be persistent in the environment. Longer term environmental effects will reflect organic materials associated with the reagent. Higher MW organic cations can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous waste streams. In addition, Polybrominated organics can be persistent and bioaccumulate.
The quaternary salts mentioned are generally tetra n-butyl, given the alkyl substituent is not directly involved it may be assumed that this could alter. Care should be taken if substitution to lower order groups particularly the tetra-methyl (Me4N+) moiety given high reported toxicity. The cation is a neurotoxin akin to paraquat and banned in the EU since 2007, recent publication; Lin CC1, Yang CC, Ger J, Deng JF, Hung DZ ClinToxicol (Phila). 201048(3):213-7 - Cost, availability & sustainable feedstocks
Most bromination reagents are available at scale with varying degrees of cost – the most economical being reagents based on Br2, HBr or simple salts like NaBr. - Sustainable implications
No real sustainability issues with bromine.
Relevant scale up example
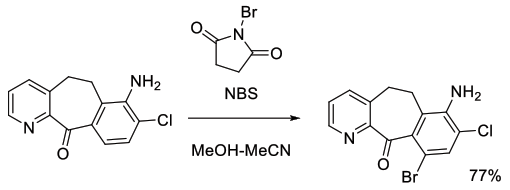
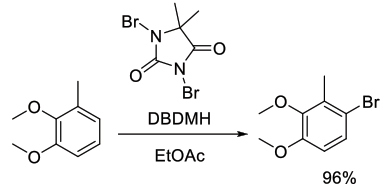
Org. Process Res. Dev., 2003, 7, 692-695
Experimental
77 kg scale
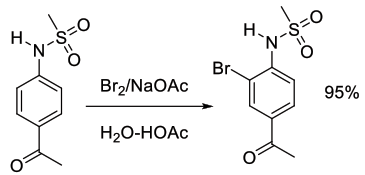
Org. Process Res. Dev., 2004, 8, 624-627
Experimental
10 kg scale
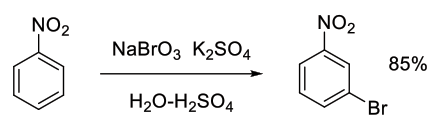
Org. Process Res. Dev., 1998, 2, 71-77
Experimental
40 kg scale
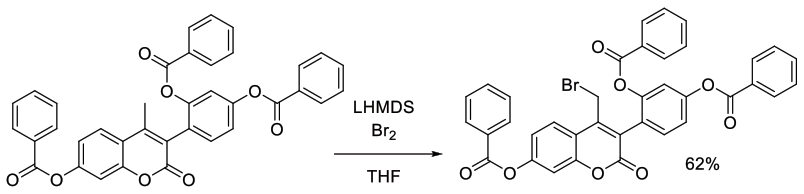
Org. Process Res. Dev., 2000, 4, 30-33
Experimental
124 g scale
Org. Process Res. Dev., 2006, 10, 354-360
Experimental
20 g scale
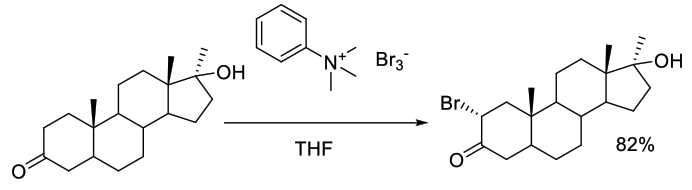
Org. Process Res. Dev., 2007, 11, 378-388
Experimental
650 g scale
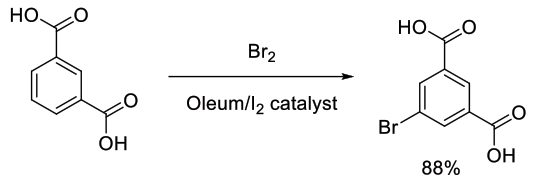
Org. Process Res. Dev., 2002, 6, 591-596
Experimental
200 g scale
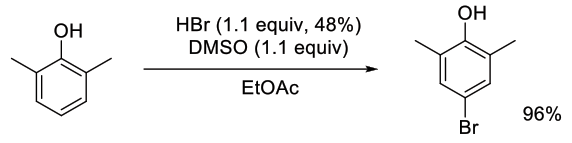
Org. Lett., 2015, 17, 2886-2889
Experimental
1 kg scale
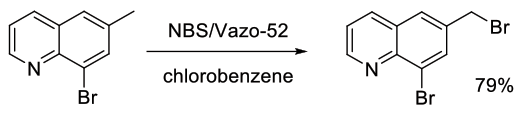
Org. Process Res. Dev., 2006, 10, 36-45
Experimental
4 kg scale
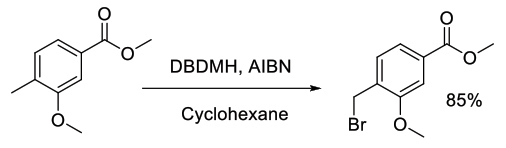
Org. Process Res. Dev., 2009, 13, 67–72
Experimental
5.4 kg scale
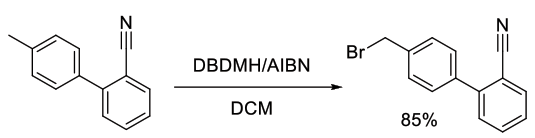
Org. Process Res. Dev., 2012, 16, 2025−2030
Experimental
150 kg scale
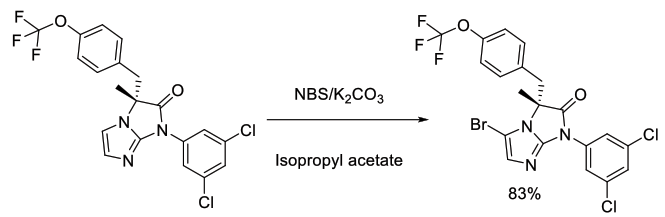
Org. Process Res. Dev., 2011, 15, 1185-1191
Experimental
1.2 kg scale
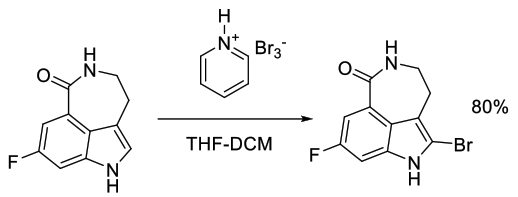
Org. Process Res. Dev., 2012, 16, 1897−1904
Experimental
6.5 kg scale
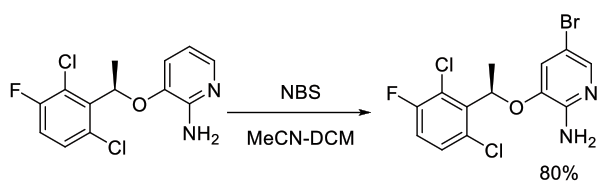
Org. Process Res. Dev., 2011, 15, 1018–1026
Experimental
43 kg scale
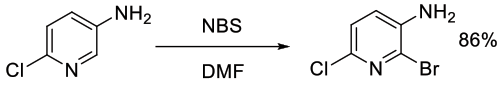
Org. Process Res. Dev., 2012, 16, 1069−1081
Experimental
8 kg scale
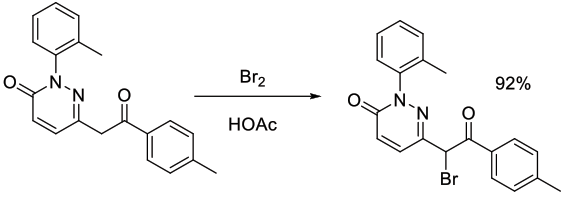
Org. Process Res. Dev., 2012, 16, 1818−1826
Experimental
29 kg scale