Deoxy Bromination
Mechanism + Description
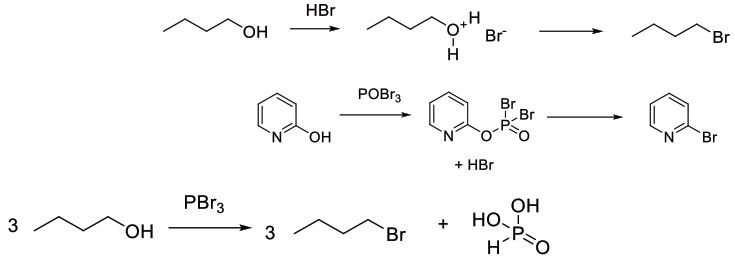
Synthesis of Carbon- Br bonds through activation of oxygen and then SN2 or SN1 displacement. Much more commonly utilized than simple bromide displacement of leaving groups.
General comments
Common reagents for deoxy bromination are SOBr2, PBr3,PBr5 POBr3 and HBr. Other less common reagents are Ph3PBr2and related P(V) reagents, which are usually made in situ from Ph3P and Br2 or CBr4 This is the bromo version of the Appel reaction.
Key references
J. Am. Chem. Soc., 1940, 62, 227–227 – The Preparation of Acetyl Bromide -Use of PBr3
J. Org. Chem., 1947, 12, 293–294 – Phosphorus Oxybromide as a brominating agent for bromopyrimidines
"Thionyl Bromide". In Paquette, L. A. – Encyclopedia of Reagents for Organic Synthesis.
Relevant scale up example
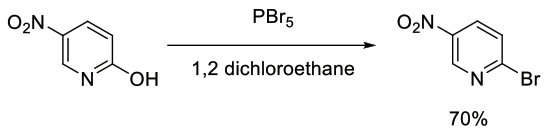
Org. Process Res. Dev., 2004, 8, 62-71
Experimental
400 g scale
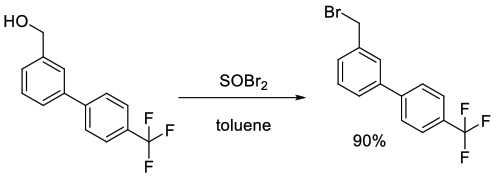
Org. Process Res. Dev., 2011, 15, 570–580
Experimental
18.5 kg scale
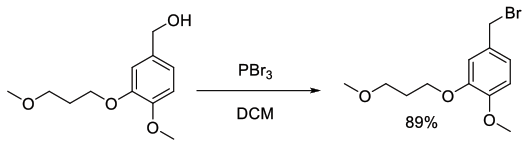
Org. Process Res. Dev., 2013, 17, 1458−1462
Experimental
60 g scale
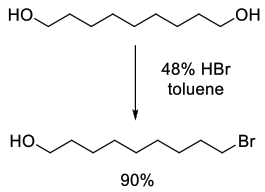
Org. Process Res. Dev., 2010, 14, 544–552
Experimental
1 kg scale

Org. Process Res. Dev., 2007, 11, 899–902
Experimental
140 kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Optimal with HBr and PBr3. Other reagents are less atom economical. The use of Ph3P and related P reagents is very atom inefficient and should be avoided if possible. Deoxybromination reagents are often used in molar excess and reactions should be optimized to use stoichiometric amounts of reagent if feasible. - Safety Concerns
Deoxybromination reactions can be very exothermic .These reagents are acidic, and will generate HBr, so material compatibility needs to be considered. Most deoxybromination reagents will react violently with water/alcohols to generate HBr. Aqueous quenching of reactions using excess PBr3, SOBr2, POBr3 can be subject to delayed exotherm due to inhomogeneity and mixing (in a similar manner to the example provided using POCl3). The eventual reaction produces a lot of heat and acidic gas. Deoxybromination reagents may show incompatibility with certain solvents.
- Toxicity and environmental/aquatic impact
Most reagents are too reactive to hydrolysis to be persistent and toxic per se. High local acidity might be an issue, but no real concerns with low levels of bromide anions following neutralisation. Longer term environmental effects will reflect organic materials associated with the reagent. Higher MW organic cations (quats, ionic liquids etc) can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous waste streams.
Ph3P and Ph3PO are of particular environmental concern and as such should not be discharged into aqueous waste streams. Inorganic P-based reagents will generate phosphate on hydrolysis and due to concerns over eutrophication, there maybe local restrictions on levels that can be discharged. Polybromoorganics can be persistent and bio-accumulate.
Many examples use hazardous chlorinated solvents, historically these have been used but greener alternatives are encouraged aligned to solvent selection guides. - Cost, availability & sustainable feedstocks
Most deoxybromination reagents are available at scale with varying degrees of cost, but typically more expensive than the corresponding chlorine–based reagents. - Sustainable implications
No sustainability implications for bromine reagents.