Nuclophilic I– Reagents
Mechanism + Description
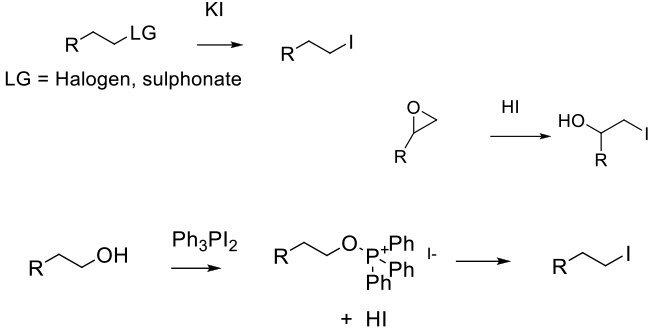
Addition of I- through reaction with an electrophile with appropriate leaving group (SN2) or activated by protonation. Alcohols can be activated in situ (e.g., Ph3PI2)
General comments
Iodine can be introduced by the reaction of iodide with appropriate nucleopiles like alkyl halides or sulphonates. With Cl- and Br- leaving groups, the reaction often has to be driven in the direction of the iodide by using the preferential solubility of NaI in ketones, acetone, (Finkelstein reaction) since I- is the best leaving group.
I- can also be inserted via metal-catalyzed halogen exchange or the classical decomposition of aryl diazonium salts (Sandmeyer reaction) in the presence of Cuprous iodide and HI.
Key references
Relevant scale up example
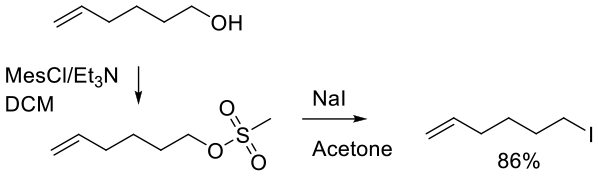
Org. Synth. 2005, 81, 121.
Experimental
20 g scale
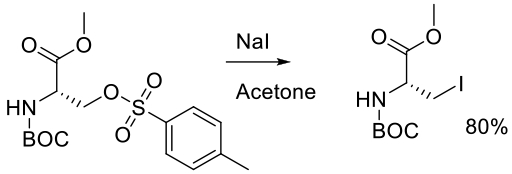
Org. Synth. 2005, 81, 77.
Experimental
27 g scale
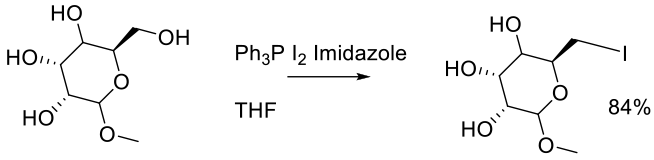
Org. Process Res. Dev. 2014, 18, 1728−1739.
Experimental
90 g scale
Green Review
-
Atom efficiency (by-products Mwt)
Optimal with simple leaving groups (Cl, Br, Mes) and salts like Na or KI. The use of Ph3P and related P reagents is very atom inefficient. - Safety Concerns
No general concerns; the unstable/explosive properties of aryl diazonium salts need consideration in use. - Toxicity and environmental/aquatic impact
High concentrations of I- reagents are toxic to aquatic organisms, especially freshwater organisms. Longer-term environmental effects will reflect organic materials associated with the reagent. Higher mol wt organic cations (quats, ionic liquids, etc.) can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous wastes. Ph3P and Ph3PO are of particular environmental concern and should not be discharged into aqueous waste streams.
Iodinated organics can be persistent and bioaccumulate.
Many examples use hazardous chlorinated solvents: Historically, these have been used, but greener alternatives aligned to solvent selection guides are encouraged. - Cost, availability & sustainable feedstocks
Most iodide reagents are available at scale with varying degrees of cost – the most economical being reagents based on HI and simple iodide salts. Na and K salts are preferred reagents. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium-to-high risk of depletion. High LCA reagents, although it is possible to recover iodide from inorganic and organic waste streams.