Iodocyclohexane
Mechanism + Description
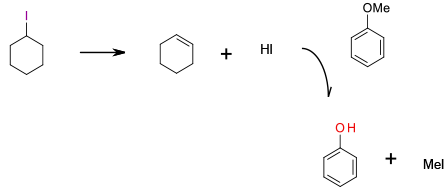
Elimination generates HI then standard SN2 cleavage of methyl ether catalysed by HI present in the reaction mixture.
General comments
Iodocyclohexane has been reported as a mild reagent for dealkylation of anisoles. This reaction requires a large excess of iodocyclohexane, with the dealkylation actually promoted by HI generated by thermal elimination with subsequent formation of cyclohexene. Large excess of an organoiodine and generation of HI waste limit the sustainability of this method.