Phosphine Reagents
Mechanism + Description
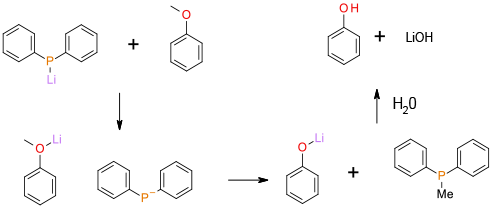
The alkyl group is cleaved by an SN2 mechanism. The lithium ion coordinates to the ether oxygen increasing the leaving group ability of the phenoxide.
General comments
Li Ph2P has been used as a nuclophile to dealkylate anisoles and related compounds. The reaction takes place under mild conditions but has the disadvantage of poor atom efficiency and reagents/by-products that could be ecotoxic. A plus point is that high degrees of selectivity are possible in complex molecules and the by-products are non-volatile and not classified as PGI’s.
Key references
Journal of the Chemical Society, 1965, 4120 The dealkylation of alkyl aryl ethers and sulfides by diaryl phosphide and arsenide ions
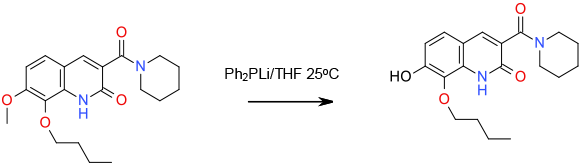
Relevant scale up example
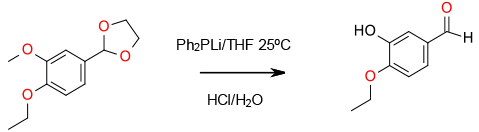
Experimental
Gram scale
Organic Syntheses, 1988, 6, 567; 1977, 44