Borane & Borane Complexes
Mechanism + Description
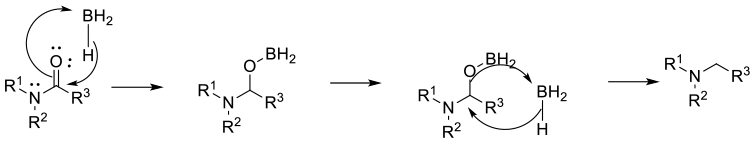
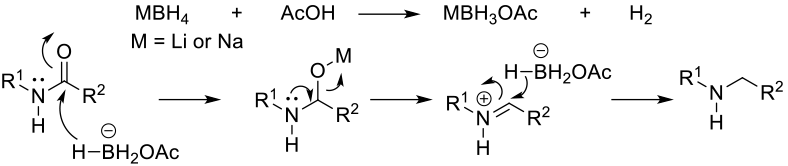
Mechanism involves activation of the carbonyl by Lewis acidic boron followed by hydride transfer, then cleavage of the C-O bond. The reaction is driven by the high affinity of Boron for Oxygen. Diborane, borane and borane complexes all proceed by essentially the same mechanism. Generally, NaBH4 will not reduce amides, but will in the presence of acids.
General comments
Generally BH3/B2H6 is too reactive and hazardous to be generated and used outside of specialised facilities, but BH3 complexes like BH3-THF, BH3-Me2S can be purchased or prepared in situ. Issues with Me2S generation can be overcome with higher Mol.Wt sulphides at the expense of atom economy, but some sulphide carriers can be recovered and reused. Amine complexes of borane are quite stable and do not reduce amides. The initial product of an amide reduction with BH3 is the amine-BH3 complex which has to be decomposed to liberate the free amine product.
NaBH4 is the usual source of borane complexes and can generate active amide reducing species in the presence of acids, Lewis acids (AlCl3, BF3OEt2) and some metal salts. Borane reagents generally show better functional group tolerance than the reactive Aluminium hydride reagents, but will reduce alkenes, acids, esters etc. Borane complexes will reduce 1°, 2°, and 3° amides, lactams And imides. Lithium aminoborohydrides are more stable than BH3 complexes and much less pyrophoric, but only reduce tertiary amides and lactams.
Key references
http://www.organic-chemistry.org/chemicals/reductions/boranes.shtm
Relevant scale up example
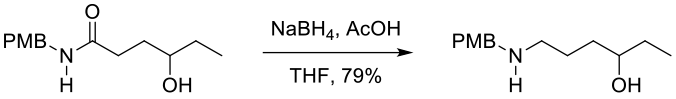
Experimental
45 kg scale
Org. Process Res. Dev. 2001, 5, 575-580.

Experimental
3 kg scale
Org. Process Res. Dev. 2011, 15, 1138-1148.

Experimental
600 g scale
Org. Process Res. Dev. 2012, 16, 499-506
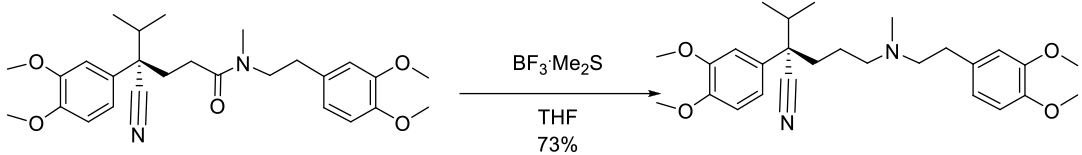
Experimental
100 g scale
Org. Process Res. Dev. 2000, 4, 467-472.
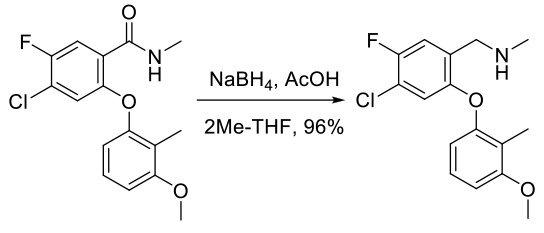
Experimental
100 g scale
Org. Process Res. Dev. 2012, 16, 1805−1810
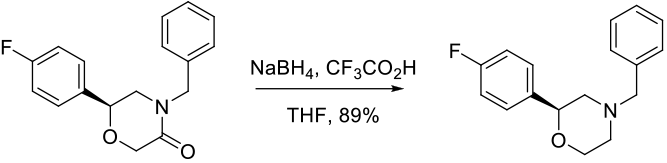
Experimental
35 kg scale
Org. Process Res. Dev. 2015 asap
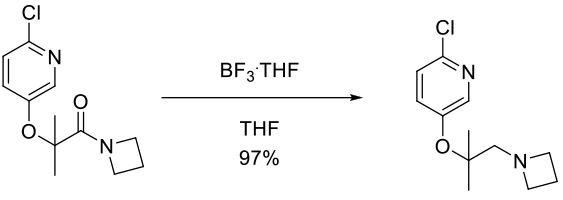
Experimental
700 g scale
Org. Process Res. Dev. 2013, 17, 876−880
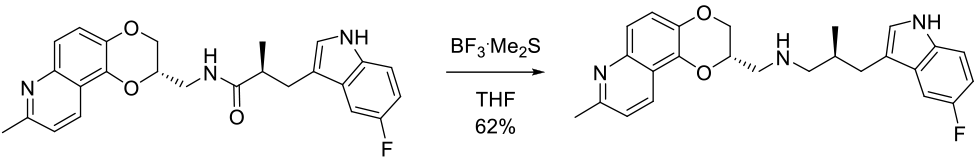
Experimental
35 g scale
Org. Process Res. Dev. 2009, 13, 91–97
Green Review
-
Atom efficiency (by-products Mwt)
- NaBH4/AcOH
By-products: 2 eq NaOH (40 g/mol) + 2 eq BH2OAc (72 g/mol) + 2 eq H2 gas (2 g/mol) = 228 g by-products/mol amide - Borane
By-products: B(OH)3 (62 g/mol) and H2 gas (2 g/mol) if quenched with water, B(OMe)3 (104 g/mol) and H2 gas (2 g/mol) if quenched with MeOH = 128+ g by-products/mol amide (depends upon quench)
- NaBH4/AcOH
- Safety Concerns
Main issues are around toxicity, flammability, safe handling and storage. Care needs to be exercised with borane complexes like BH3THF that concentrated solutions are avoided. In some cases above ~ 1M there can be considerable dissociation of BH3 forming an atmosphere of B2H6 in the reaction headspace. Hydrogen may be generated during reaction and work-up. Anionic borane complexes are more stable. There maybe issues with some solvents commonly used in borane chemistry – see later. - Toxicity and environmental/aquatic impact
Boranes highly toxic to mammals. Hydrolysis of Boron-based reagents will lead to boric acid which is a suspected reprotoxic mutagen. There may be issues with discharging aqueous waste with high B content. Emerging data suggest Boron compounds maybe more ecotoxic that previously thought.
Chemosphere. 2011 Jan;82(3):483-7. Effects assessment: boron compounds in the aquatic environment. - Cost, availability & sustainable feedstocks
Borane reagents can be purchased in bulk or made in situ at reasonable cost. There is no renewable source of borane reagents. - Sustainable implications
No great issues with Boron. With anionic complexes, Na and K are preferred cations -Li is at medium risk of depletion. Some reactions now being reported in biorenewable solvents like 2-methyl THF.