Catalytic Hydrogenation
Mechanism + Description
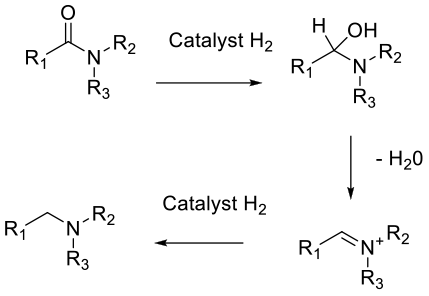
Can be via hetero or homogeneous metal catalysis and H2 Typically a reduction to the hemi-aminal occurs followed by elimination of water to an imine or iminium species which is then hydrogenated to the amine product. A side reaction is the alcohol formed by elimination of the amine from the hemi-aminal. Heterogeneous catalysts can reduce aromatic rings.
General comments
Potentially the greenest route to amines from amides, but traditionally not widely used in the pharmaceutical industry due to harsh conditions and specialist equipment – high temp, high H2 pressures. Poor selectivity and side reactions can also lower yields. Recently a number of groups have developed much more active catalysts (mainly homogeneous) that show high selectivity for the amine under milder conditions. Primary and secondary amides are preferred substrates, tertiary amides are possible, but problematic. Key academic groups working in this area are listed below. The advances in identifying more active and selective catalysts will make this route to amines more attractive for pharma synthesis.
David Cole-Hamilton
Robert Crabtree
David Milstein
Takao IKARIYA
Matthias Beller
Key references
J.Am. Chem. Soc. 1934, 56, 2419–2424 Catalytic Hydrogenation of Amides to Amines
Relevant scale up example
None found.