Organocatalysis & Metal Free Reduction
Mechanism + Description
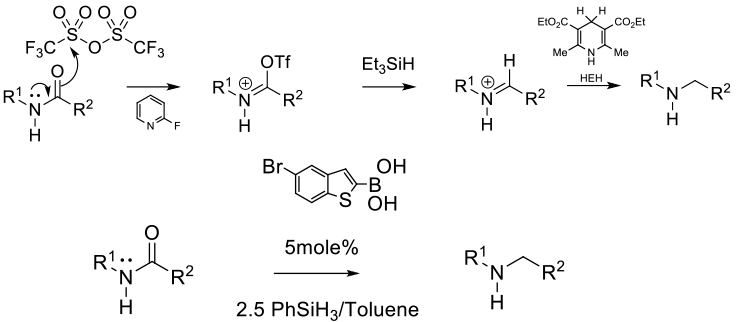
A variant on metal catalysed hydrosilyation in which Hantzsch esters and silanes are used as reductants. Prior activation of the sec or tert amide as the imidoyl triflate is required. More reactive catalysts like boronic acids do not require pre-activation of the amide.
General comments
A number of reductants will reduce activated secondary and tertiary amides – historically as imidoyl triflates, but later work, especially using boronic acids as catalysts with PMHS, TMDS and PhSiH3 will reduce inactivated primary, sec and tert amides. Generally mild conditions and good functional group tolerance, but poor atom economy especially if excess high Mwt silanes / stoichiometric Hantzsch esters are needed as reductants.
Atom Efficiency
- Tf2O/ Hantzsch esters
By-products: 2 eq TfO- (149 g/mol) + 2 eq HEH (253 g/mol) = 804 g by-products/mol amide - Tf2O/Et3SiH/ Hantzsch esters
By-products: 2 eq TfO- (149 g/mol) + 1 eq 2-Fpyr (97 g/mol) + 1 eq Et3Si (115 g/mol) + 1 eq HEH (253 g/mol) = 763 g by-products/mol amide
Generally better atom efficiency is obtained with boronic acid catalysts and silanes avoiding triflic anhydride which is highly corrosive and the by-product trific acid is a persistent environmental pollutant.
Key references
J.Am. Chem. Soc. 2010, 132, 12817-12819. Controlled and Chemoselective Reduction of Secondary Amides
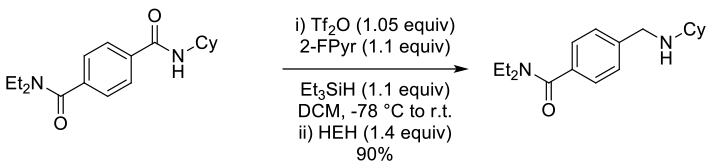
Sample Reaction: (up to 1g substrate)