Nuclophilic Br- Reagents
Mechanism + Description
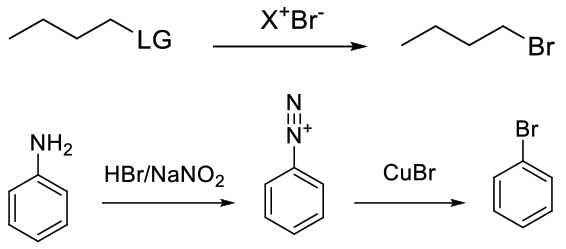
Typically SN2 with displacement of another halogen (iodide) or other leaving group (sulphonate) etc. Also via reaction of Br with other electrophiles such as diazonium salts.
General comments
Normally simple bromide salts like Na/K/Li bromide are used, or tetraalkyl ammonium or phosphonium bromides like n-Bu4NBr. Hydrogen bromide is typically used for deoxybromination, but can be used to add to unsaturated C-C bonds or ring-open epoxides etc.
Key references
“Sodium Bromide” In Paquette, L. A. Encyclopedia of Reagents for Organic Synthesis
Synthesis, 2007, 2534-2538 – Catalytic SandmeyerBromination
Relevant scale up example
None located.
Specific solvent issues with bromination
Many radical and electrophilc brominations have traditionally been carried out in chlorinated solvents – CCl4, CHCl3, C2H4Cl2 and CH2Cl2. – if a chlorinated solvent is needed, CH2Cl2 should be used.
Radical brominations with Br2 or NBS/DBDMH were traditionally carried out in CCl4, C6H6, CHCl3. A range of alternative solvents for radical bromination has been reported. Some alternatives to the above solvents are listed below.
Chlorobenzene, benzotrifluoride, n-hexane – less favoured
Cyclohexane, heptane, Dimethyl carbonate, MeCN, methyl acetate isopropyl acetate – favoured
Bromine, Br+ reagents and higher oxidation state Br compounds are oxidising reagents and can react violently with some organic solvents and reagents, especially ethers if traces of peroxides are present. The use of DMSO in the presence of Br2 or HBr should be treated with caution.
Unexpected reaction between NBS and NMP
DMSO – Technical information package
An explosion has been reported by an unexpected reaction of acetone with POCl3 POBr3 would probably show similar instability.