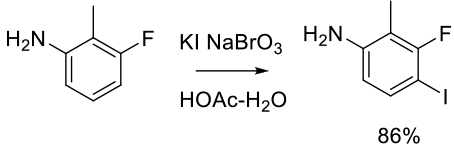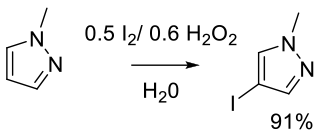Mechanism + Description
I2 and I+ equivalents are electrophilic reagents normally used to add to unsaturated C-C bonds, take part in electrophilic substitution reactions with aromatics/heteroaromatics, or react with acidic C-H bonds. The attacking species is often written as I2 or I+, but may be more complex than these simple species.
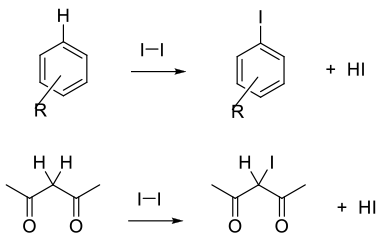
General comments
I2 is the weakest electrophile of the molecular halogens, but will react directly with electron-rich double bonds. It is often activated by strong acids, Lewis acids, and oxidants. Oxidants can boost the iodination power of the reagent and can also generate I2 / I+ in situ from HI or simple iodide salts. Oxidants can also reoxidize any I– produced and maximize the use of iodine. Electrophilic iodination in the presence of chlorine reagents/chloride can occasionally result in chlorinated materials as by-products. Common oxidants and acid activators are listed below.
Common (re)oxidants for iodination:
H2O2 or urea H2O2 complex
AgNO3/Ag2SO4
NaBrO3
Ammonium peroxodisulfate (NH4)2S2O8
Tetrabutylammonium peroxydisulfate (nBu4N)2S2O8
Oxone KHSO5 0.5KHSO4 0.5K2SO4
Chloramine T
Selectfluor F−TEDA−BF4
NaOCl
KIO3/NaIO4
Common acid activators for iodination:
H2SO4
p-Tosic acid
CF3SO3H
MeSO3H
CF3CO2H
Other electrophilic iodination reagents are N-I reagents like NIS and DIDMH (1,3-diiodo-5,5-dimethylhydantoin). N-iodosuccinimide (NIS) is an expensive reagent and is often replaced by N-chlorosuccinimide (NCS), plus an iodine source. Other common reagents are ICl and related complexes, and anionic ICl2– reagents.
Less reactive (electrophilic) substrates may need conversion to anions prior to reaction with I+ reagent. The choice of which I+ reagent to use comes down to screening for yield and required selectivity, avoiding polyiodination and unwanted oxidation side reactions.
Key references
Shen, H.; Vollhart, P. C. Remarkable Switch in the Regiochemistry of the Iodination of Anilines by N-Iodosuccinimide: Synthesis of 1,2-Dichloro-3,4-diiodobenzene. Synlett. 2012, 2, 208-214.
Bovonsombat, P.; Leykajarakul, J.; Khan, C.; Pla-on, K.; Krause, M. M.; Khanthapura, P.; Ali, R.; Doowa, N. Regioselectiveiodination of phenol and analogues using N-iodosuccinimide and p-toluenesulfonicacid. Tetrahedron Lett. 2009, 50, 2664-2667.
Olah, G. A.; Wang, Q.; Sandford, G.; Parkash, G. K. S. Synthetic methods and reactions. 181. Iodination of deactivated aromatics with N-iodosuccinimide in trifluoromethanesulfonic acid (NIS-CF3SO3H) via in situ generated superelectrophilic iodine(I) trifluoromethanesulfonate. J. Org. Chem. 1993, 58(11), 3194–3195.
Suryankiran, N.; Reddy, T. S.; Suresh, V.; Lakshman, M.; Venkateswarlu, Y. Synthesis of α-iodoβ-ketosulfones and α-iodomethylsulfones using iodine monochloride. Tetrahedron Lett. 2006, 47(26), 4319–4323.
Kim, M. M.; Ruck, R. T.; Zhao, D.; Huffman, M. A. Green iodination of pyrazoles with iodine/hydrogen peroxide in water. Tetrahedron Lett. 2008, 49(25), 4026–4028.
Kraszkiewicz, L.; Sosnowski, M.; Skulski, L. Oxidative Iodination of Deactivated Arenes in Concentrated Sulfuric Acid with I2/NaIO4 and KI/NaIO4 Iodinating Systems. Synlett. 2006, 7, 1195-1199.
Castanet, A.; Colobert, F.; Broutin, P. Mild and regioselective iodination of electron-rich aromatics with N-iodosuccinimideand catalytic trifluoroaceticacid. Tetrahedron Lett. 2002, 43(29), 5047-5048.
Mohanakrishnan, A. K.; Prakash, C.; Ramesh, N. A simple iodination protocol via in situ generated ICl using NaI/FeCl3. Tetrahedron. 2006, 62(14), 3242-3247.
Pavlinac, J.; Zupan, M.; Stavber, S. ‘Green’ Iodination of Dimethoxy- and Trimethoxy-Substituted Aromatic Compounds Using an Iodine-Hydrogen Peroxide Combination in Water. Synthesis. 2006, 15, 2603-2607.
Hajipour, A. R.; Arbabian, M.; Ruoho, A. E. TetramethylammoniumDichloroiodate: An Efficient and Environmentally Friendly Iodination Reagent for Aromatic Compounds under Mild and Solvent-Free Conditions. J. Org. Chem. 2002, 67(24), 8622-8624.
Khansole, S. V.; Junne, S. B.; Sayyed, M. A.; Vubhute, Y. B. Convenient and Efficient Method for the Iodination of Aromatic Amines by PyridiniumIodochloride. Synthetic Communications. 2008, 38(11), 1792 – 1798.
D’Auria, M.; Mauriello, G. Reevaluationof BenzyltrimethylammoniumDichloroiodide, Previously Reported to be a Selective Iodinating Agent. Synthesis. 1995, No. 3, 248-250.
Tilve, R. D.; Kanetkar, V. R. RegioselectiveIodination of Activated Arenes Using Phenyl TrimethylammoniumDichloroiodate in Ionic Liquid Under Microwave Irradiation. Synthetic Communications. 2005, 35(10), 1313-1318.
Garden, S. J.; Torres, J. C.; De Souza Melo, S. C.; Lima, A. S.; Pinto, A. C.; Lima, E. L. S. Aromatic iodination in aqueous solution. A new lease of life for aqueous potassium dichloroiodate. Tetrahedron Lett. 2001, 42(11), 2089-2092.
Kosynkin, D. V.; Tour, J. M. BenzyltriethylammoniumDichloroiodate/Sodium Bicarbonate Combination as an Inexpensive, Environmentally Friendly, and Mild Iodinating Reagent for Anilines. Org. Lett. 2001, 3(7), 991–992.
“For BenzyltriethylammoniumDichloroiodate iodination of a substituted phenol”: Shotwell, J. B.; Krygowski, E. S.; Hines, J.; Koh, B.; Huntsman, E. W. D.; Choi, H. W.; Schneekloth, J. S., Jr.; Wood, J. L.; Crews, C. M. Total Synthesis of Luminacin D. Org. Lett. 2002, 4(18), 3087–3089.
Reddy, K. S. K.; Narender, N.; Rohitha, C. N.; Kulkarni, S. J. Iodination of Aromatic Compounds Using Potassium Iodide and Hydrogen Peroxide. Synthetic Communications. 2008, 38(22), 3894-3902.
Ganguly, N. C.; Barik, S. K.; Dutta, S. EcofriendlyIodination of Activated Aromatics and Coumarins Using Potassium Iodide and Ammonium Peroxodisulfate. Synthesis. 2010, No. 9, 1467-1472.
Filimonov, V. D.; Semenischeva, N. I.; Krasnokutskaya, E. A.; Hwang, H. Y.; Chi, K. TetraalkylammoniumDichloroiodates as Iodinating Agents: Absence of Activity in Solid Phases and Superelectrophilic Activity in Sulfuric Acid. Synthesis. 2008, 3, 401-404.
Da Ribeiro, R.S.; Esteves, P.M.; de Mattos, M.C.S. SuperelectrophilicIodination of Deactivated Arenes with TriiodoisocyanuricAcid. Synthesis. 2011, No. 5, 739-744.
Barluenga, J.; Marco-Arias, M.; González-Bobes, F.; Ballesteros, A.; González, J. M. New reactions in water: metal-free conversion of alcohols and ketones into α-iodoketones with NaI H2O2 H+. Chem. Commun. 2004, 2616-2617.
Gallo, R. D. C.; Ferreira, I. M.; Casagrande, G. A.; Pizzuti, L.; Oliveira-Silva, D.; Raminelli, C. Efficient and eco-friendly synthesis of iodinated aromatic building blocks promoted by iodine and hydrogen peroxide in water: a mechanistic investigation by mass spectrometry. Tetrahedron Lett. 2012, 53(40), 5372–5375.
Pavlinac, J.; Zupan, M.; Stavber, S. Effect of Water on the Functionalization of Substituted Anisoles with Iodine in the Presence of F−TEDA−BF4 or Hydrogen Peroxide. J. Org. Chem. 2006, 71(3), 1027–1032.
Edgar, K. J.; Falling, S. N. An efficient and selective method for the preparation of iodophenols. J. Org. Chem. 1990, 55(18), 5287–5291.
Adimurthy, S.; Ramachandraiah, G.; Ghosh, P. K.; Bedekar, A. V. A new, environment friendly protocol for iodination of electron-rich aromatic compounds. Tetrahedron Lett. 2003, 44, 5099–5101.
Iskra, J.; Stavber, S.; Zupan, M. Nonmetal-CatalyzedIodination of Arenes with Iodide and Hydrogen Peroxide. Synthesis. 2004 , No. 11, 1869-1873.
Krasovskiy, A.; Krasovskaya, V.; Knochel, P. Mixed Mg/Li Amides of the Type R2NMgCl⋅LiCl as Highly Efficient Bases for the Regioselective Generation of Functionalized Aryl and Heteroaryl Magnesium Compounds. Angewandte Chemie International Edition. 2006, 45(18), 2958-2961.
Kometani, T.; Watt, D. S.; Ji, T. Iodination of phenols using chloramine T and sodium iodide. Tetrahedron Lett. 1985, 26(17), 2043-2046.
Narender, N.; Srinivasu, P.; Kulkarni, S. J. RegioselectiveOxyiodination Of Aromatic Compounds Using Potassium Iodide And Oxone®. Synthetic Communications. 2002, 32, 2319-2324.
Kalyani, D.; Dick, A. R.; Anani, W. Q.; Sanford, M. S. A Simple Catalytic Method for the Regioselective Halogenation of Arenes. Org. Lett. 2006, 8(12), 2523-2526.
“For Heteroaryl iodides from lithiatedheterocycles”: Estel, L.; Marsais, F.; Queguinar, G. Metalation/SRN1 coupling in heterocyclic synthesis. A convenient methodology for ring functionalization. J. Org. Chem. 1988, 53(12), 2740–2744.
Lulinski, P.; Kryska, A.; Sosnowski, M.; Skulski, L. Eco-friendly Oxidative Iodination of Various Arenes with a Urea-Hydrogen Peroxide Adduct (UHP) as the Oxidant.Synthesis. 2004, 3, 441-445.
Yang, S. G.; Kim, Y. H. A practical iodination of aromatic compounds using tetrabutylammoniumperoxydisulfate and iodine. Teterhedron Lett. 1999, 40(33), 6051-6054.
Whang, J. P.; Yang, S. G.; Kim, Y. H. Novel α-iodination of functionalized ketones with iodine mediated by bis(tetra-n-butylammonium) peroxydisulfate. Chem. Commun. 1997, 1355-1356.
Yusubov, M. S.; Tveryakova, E. N.; Krasnokutskaya, E. A.; Perederyna, I. A.; Zhdankin, V. V. Solvent‐Free Iodination of Arenes using Iodine–Silver Nitrate Combination. Synthetic Communications. 2007, 37(8), 1259-1265.
Castanet, A.; Colobert, F.; Broutin, P. Mild and regioselective iodination of electron-rich aromatics with N-iodosuccinimide and catalytic trifluoroaceticacid. Tetrahedron Lett. 2002, 43(29), 5047-5048.
Leboeuf, D.; Ciesielski, J.; Frontier, A. J. Gold(I)-Catalyzed Iodination of Arenes. Synlett. 2014, 25(3), 399-402.
Jakab, G.; Hosseini, A.; Hausmann, H.; Schreiner, P. R. Mild and Selective Organocatalytic Iodination of Activated Aromatic Compounds. Synthesis. 2013, 45(12), 1635-1640.
Thiebes, C.; Prakash, G. K. S.; Petasis, N. A.; Olah, G. A. Mild Preparation of Haloarenes by Ipso-Substitution of Arylboronic Acids with N-Halosuccinimides. Synlett. 1998, 2, 141-142.
Du, B.; Jiang, X.; Sun, P. Palladium-CatalyzedHighly Selective ortho-Halogenation (I, Br, Cl) of Arylnitrilesvia sp2 C-H Bond Activation Using Cyano as Directing Group. J. Org. Chem. 2013, 78(6), 2786-2791.
Relevant scale up example
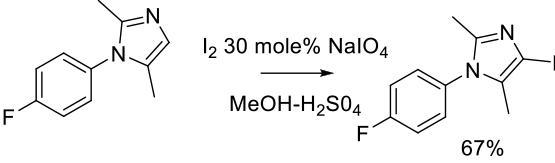
Org. Process Res. Dev. 2011, 15, 449–454.
Experimental
100 g scale
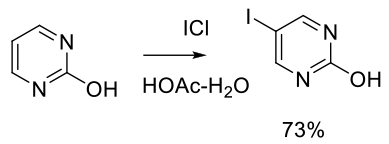
Org. Process Res. Dev. 2007, 11, 237-240.
Experimental
100 g scale
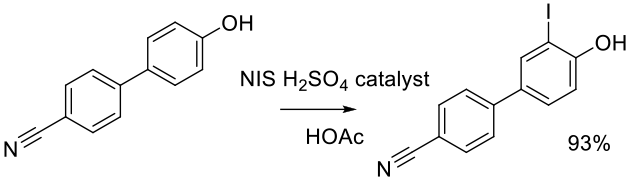
Org. Process Res. Dev. 2001, 5, 80-83.
Experimental
2.2 kg scale
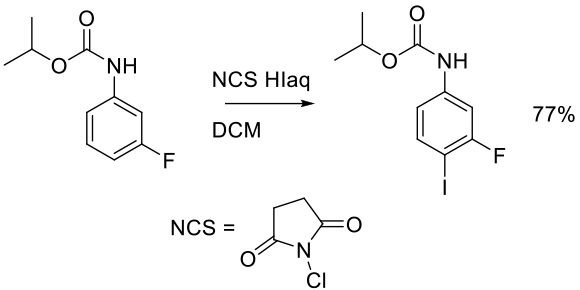
Org. Process Res. Dev. 2001, 5, 80-83.
Experimental
50 g scale
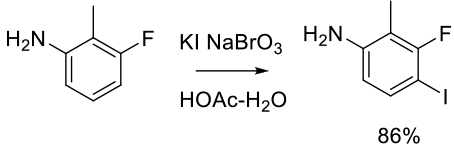
Org. Process Res. Dev. 2012, 16, 561−566.
Experimental
400 g scale
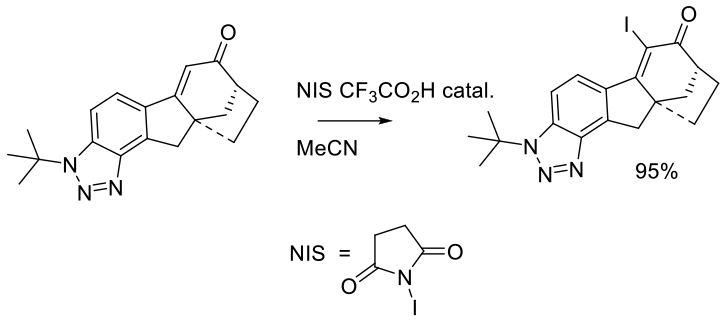
Org. Process Res. Dev. 2014, 18, 528−538.
Experimental
27 kg scale
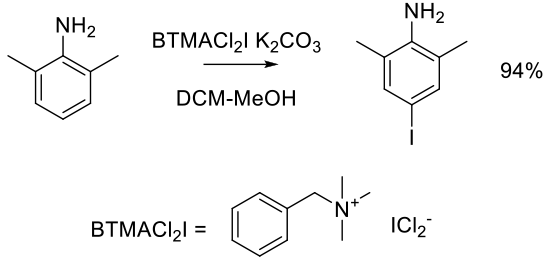
Org. Process Res. Dev. 2012, 16, 1953−1966.
Experimental
68 kg scale
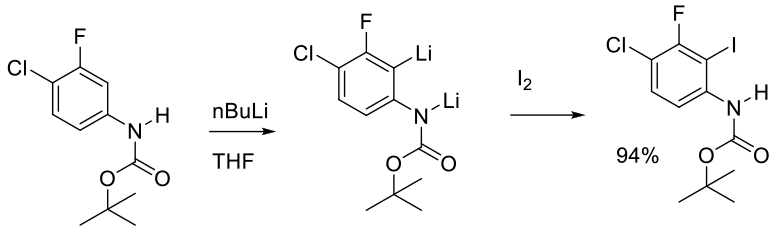
Org. Process Res. Dev. 2010, 14, 1248–1253.
Experimental
80 g scale
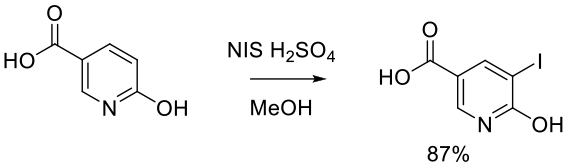
Org. Process Res. Dev. 2013, 17, 940−945.
Experimental
80 g scale
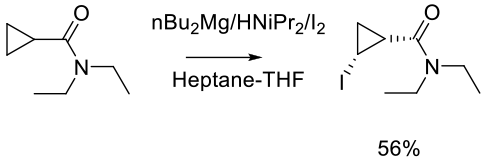
Org. Process Res. Dev. 2004, 8, 353-359.
Experimental
50 g scale
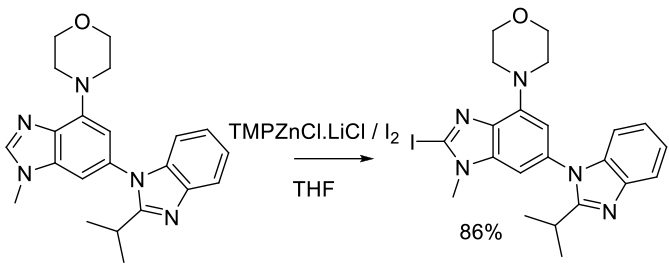
Org. Process Res. Dev. 2013, 17, 138−144.
Experimental
120 g scale
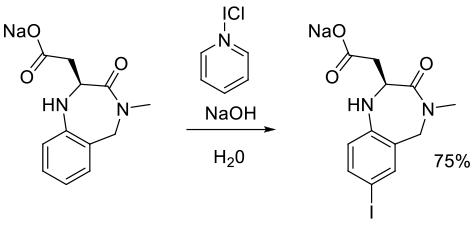
Org. Process Res. Dev. 2003, 7, 663-675.
Experimental
120 kg scale
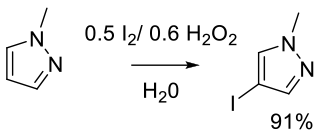
Org. Process Res. Dev. 2012, 16, 1329−1337.
Experimental
7 kg scale
Org. Process Res. Dev. 2011, 15, 1149–1162.
Experimental
125g scale
Green Review
-
Atom efficiency (by-products Mwt)
HI and I2 with activators/(re)oxidants are the most atom-efficient and maximize the use of Iodine.
- Safety Concerns
N-I and hypervalent Iodine reagents can be explosive, and many iodination reactions run with iodide and oxidants can be highly exothermic and evolve gas (O2).
- Toxicity and environmental/aquatic impact
High concentrations of I+ reagents will be extremely toxic to aquatic organisms, but are probably too reactive to be persistent in the environment. Longer-term environmental effects will reflect organic materials associated with the reagent. Higher Mwt organic cations can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous wastes.
Iodinated organics can be persistent and bioaccumulate.
The quaternary salts mentioned are generally tetra n-butyl; given the alkyl substituent is not directly involved, it may be assumed that this could alter. Care should be taken if substitution to lower order groups – particularly the tetra-methyl (Me4N+) moiety – given high reported toxicity. The cation is a neurotoxin akin to paraquat and banned in the EU since 2007:
Tetramethylammoniumhydroxide poisoning. Lin, C. C.; Yang, C. C,; Ger, J.; Deng, J.; Hung, D. Clin. Toxicol (Phila). 2010, 48(3), 213-217.
- Cost, availability & sustainable feedstocks
Most iodination reagents are available at scale with varying degrees of cost, the most economical being reagents based on HI, I2, or simple Iodide salts with oxidants/activators.
- Sustainable implications
Incineration of waste streams can be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium-to-high risk of depletion. High LCA reagents, although it is possible to recover iodide from inorganic and organic waste streams.