Acids
Mechanism + Description
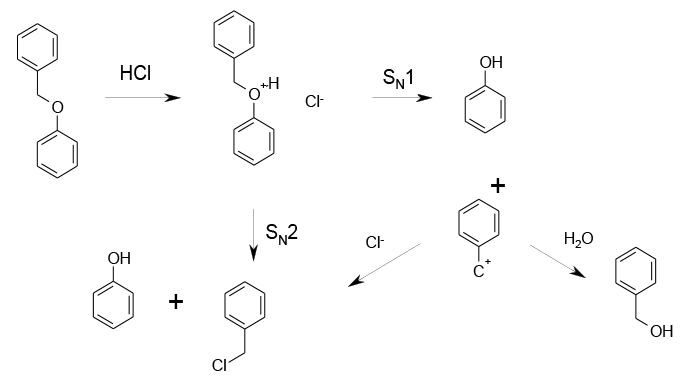
Typical SN2 or SN1 depending on substrate. Cleavage by SN1 generating a cation may give other products than the corresponding halide such as alcohols, ethers, alkenes etc.
General comments
A wide range of acids has been reported in the cleavage of acetals, ethers and esters. Normally groups that would cleave under SN1 conditions – benzyl, t-Bu are most common, but any ethers can be cleaved, although fairly harsh conditions maybe needed for cleaving primary alkyl ethers. It is quite common to find mixed acids and salts like pyridine. HCl being employed. Most acidic cleavage reactions will generate the corresponding alkyl halide or ester of the acid used and hence environmentally undesirable volatile by-products and PGI’s.
Key references
Cleavage of THP ethers
Org. Proc. Res. Dev., 2008, 429 Tet. Asym., 2003, 3435 EtOH-HCl
J. Am. Chem. Soc.,1978, 1942 Org. Letts., 2000, 3107 Tosic acid-MeOH
J. Org. Chem., 1979, 1438 Acetic acid
Tet. Lett., 2004, 1489 Amberlyst H-15, MeOH
Tetrahedron, 2007, 3782 Trifluoroacetic acid
Cleavage of t-Bu ethers
Org. Proc. Res. Dev., 2002, 120 HCO2H
Org. Proc. Res. Dev., 2003, 244 Trifluoroacetic acid
Clevage of benzylidine acetals
J. Am. Chem. Soc., 1950, 561 H2SO4
J. Am. Chem. Soc., 2001, 6983 J. Am. Chem. Soc.,1962, 430 Acetic acid
Org .lett. 2001, 3575 Tet. Lett. 1998, 233 Tet. Lett. 1999, 5845 Trifluoroacetic acid
General
Clevage of ethers
Synthesis, 2009, 2025 cleavage of aryl methyl ethers with MeSO3H-P4O10
J. Org. Chem., 2004, 69, 3340 Green Protocol for the Regeneration of Phenols from Ethers
Relevant scale up example
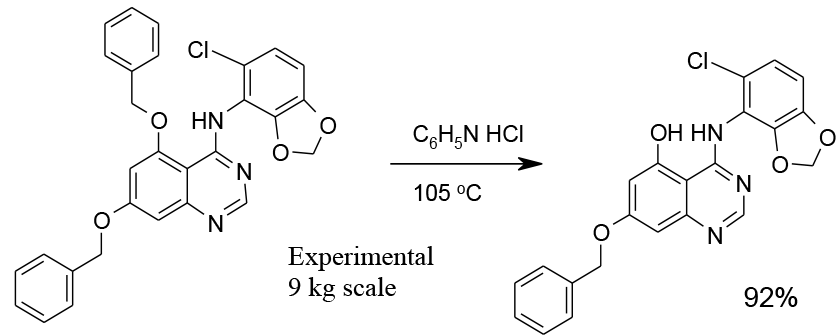
Experimental
9 kg scale
Org. Process Res. Dev., 2010, 14, 1078
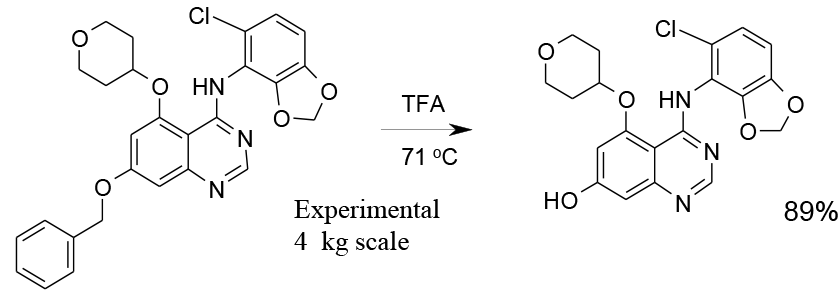
Experimental
4 kg scale
Org. Process Res. Dev., 2010, 14, 1078
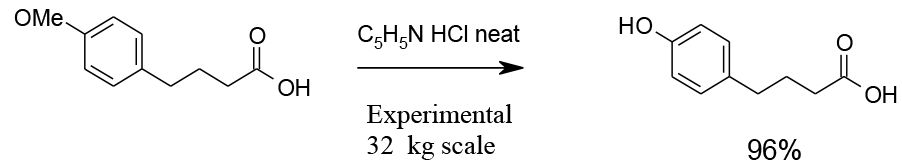
Experimental
32 kg scale
Org. Process Res. Dev., 2004, 8, 670
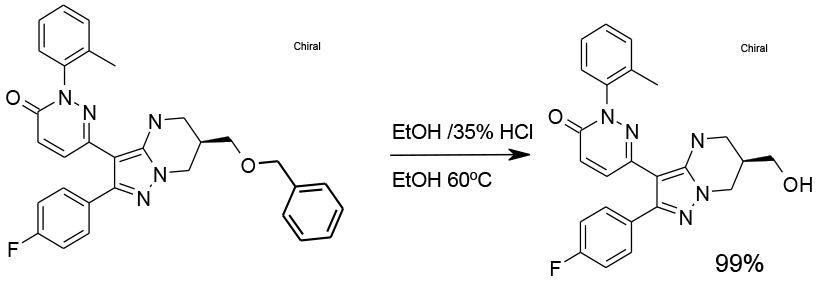
Experimental
25 kg scale
Org. Process Res. Dev., 2012, 16,1818
Green Review
-
Atom efficiency (by-products Mwt)
Dependent on acid used – most are employed in large excess as solvent. - Safety Concerns
Main issues are around corrosion and handling of strong acids. HCl and HBr may give rise to off gassing of volatile alkyl chlorides/bromides. Acid deprotection of t-Bu esters may give rise to isobutylene. - Toxicity and environmental/aquatic impact
Most mineral acids pose little problem after neutralization and dilution. High phosphate levels may be regulated due to concerns over eutrophication. Organic sulfonated acids and especially fluorinated materials can be persistent and accumulative in the environment. With all acids, alkyl derivatives of the anion can be formed. These will be positive as PGI ‘s – alkyl halides, alkyl mesylates/tosylates etc so appropriate attention needs to be given, especially if close to final API. - Cost, availability & sustainable feedstocks
Very cheap and readily available. Some organosulfonates are becoming available prepared from biorenewable sources. - Sustainable implications
Generally good with mineral acids – less so for sulfonate esters and fluorinated acids/sulfonates which will have a bigger LCI in their manufacture and more issues with persistence in the environment.