Amine Bases/Alkoxides
Mechanism + Description
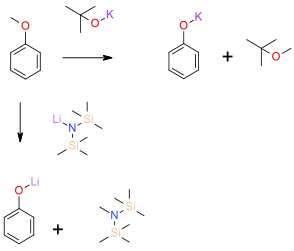
The alkyl group is cleaved by an SN2 mechanism to give metal phenoxides which are converted to phenols on quenching. The cleaved alkyl group ends up as an amine or dialkyl ether.
General comments
Sec amine bases and alkoxides in aprotic solvents can be effective reagents for the dealkylation of anisoles and related derivatives. Disadvantages are a very basic reaction medium and high temperatures (180 -190 OC) , this generally makes this methodology unattractive for many complex targets. The poor nucleophilicity of these reagents is the reason why high temperatures are needed for an acceptable reaction rate. The cleaved alkyl group ends up as the corresponding amine or bis-alkyl ether thus avoiding volatile and genotoxic by-products. Several recent publications have shown that the use of microwave heating and solvent choice can greatly lower reaction time and temperatures.
Key references
Journal of Organic Chemistry, 1997, 62, 4097 Sodium Bis(trimethylsilyl)amide and Lithium Diisopropylamide in Deprotection of Alkyl Aryl Ethers
Chemistry & Industry (London, United Kingdom) (1957), 80 Sodium amide in boiling piperidine: versatile reagent in aromatic chemistry
Relevant scale up example
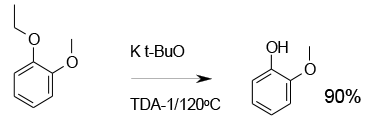
Experimental
Application of Focused Microwaves
to the Scale-Up of Solvent-Free Organic
Reactions
(TDA-1 is Tris[3,6-dioxaheptyl]amine)
Org. Process Res. Dev., 2000, 4, 498