Biocatalysis
Mechanism + Description
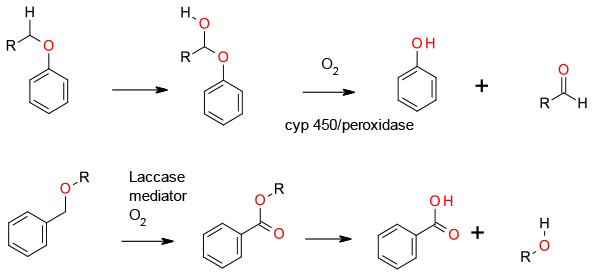
Cyp 450 and peroxidase enzymes cleave via CH oxidation followed by collapse to the phenol and aldehyde. Cyp 450’s use O2 as the terminal oxidant and peroxidases use H2O2. Laccases also give an alcohol and aldehyde product, but may go through a radical or cationic mechanism rather than oxygen insertion. Some radical pathways operate via oxidation of the ether to an ester which then hydrolyses.
General comments
A number of enzymes have activities that can lead to the cleavage of ethers – this is a common metabolic pathway for metabolism of pharmaceuticals by Cyp 450 enzymes. Generally isolated Cyp 450 enzymes are just too expensive and unproductive for preparative use other than small scale metabolite synthesis /ADME screening. Whole cell reactions can be more productive, but are still often very volume inefficient. Peroxidases present a more attractive option for scale up, but care needs to be taken with peroxide concentrations and enzyme deactivation. Laccase enzymes in combination with redox mediators can cleave ethers – oxygen being the terminal oxidant.
Key references
CYP 450/ peroxidase
FASEB Journal, 1992, 6, 686 Mechanisms of cytochrome P450 and peroxide -catalyzed xenobiotic metabolism
Current Opinion in Chemical Biology, 2011, 15, 241 Cytochromes P450: exploiting diversity and enabling application as biocatalysts
Chemistry – A European Journal, 2009, 15, 11723 A Panel of Cytochrome P450 BM3 Variants to Produce Drug Metabolites and Diversify Lead Compounds
Free Radical Biology & Medicine, 2012, 52, 340 Peroxidase-mediated dealkylation of tamoxifen
Chem. Commun., 2006, 43, 4492 Probing the substrate specificity of the catalytically self-sufficient cytochrome P450 RhF from a Rhodococcus sp
Laccase chemistry
New Journal of Chemistry, 2003, 27, 329 A study of the oxidation of ethers with the enzyme laccase under mediation by two N-OH-type compounds
European Journal of Organic Chemistry, 2002, 24, 4195 A mechanistic survey of the oxidation of alcohols and ethers with the enzyme laccase and its mediation by TEMPO
Relevant scale up example
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficiency depends on the terminal oxidant and any co-factor recycling required. Oxidases using O2 give H2O as the by-product. P450 enzymes need co-factor recycle. If using whole cells, this may be possible through cell metabolism. If not, other enzyme and / or chemical oxidants can be employed ideally the choice should be made so as to minimize any mass burden on the process. - Safety Concerns
Generally biocatalytic methods are free of thermal events and can be managed in standard equipment. - Toxicity and environmental/aquatic impact
The biocatalysts are commonly biodegradable and pose minimal environmental hazards. Some of the proteins, especially dusty solids can be sensitizing (R42, H334) so appropriate handling should be employed. For use in c-GMP manufacture of API, enzymes from mammalian sources or those fermented using mammalian products should be avoided. Older work using whole cells and biphasic systems often use dialkyl phthalates as the organic phase. These materials are persistent and endocrine disruptors hence should be avoided. - Cost, availability & sustainable feedstocks
Many enzymes are now becoming commercially available in bulk, and many CRO’s can offer biocatalysis and enzyme development services. In most cases, at the pilot stage, a biocatalysed reaction will cost more than an chemical alternative (fermentation / enzyme supply is very sensitive to economies of scale), but at full scale most biocatalysed processes are greener and cheaper than chemical alternatives. - Sustainable implications
Enzymes are produced from renewable materials and are fully biodegradable back to innocuous natural products (amino acids). The use of modern molecular biology and fermentation technology has greatly reduced the LCI of enzyme manufacture. Maximum sustainability benefits are usually obtained with mutant recombinant enzymes rather than natural enzymes.