Boron/Aluminum Reagents
Mechanism + Description
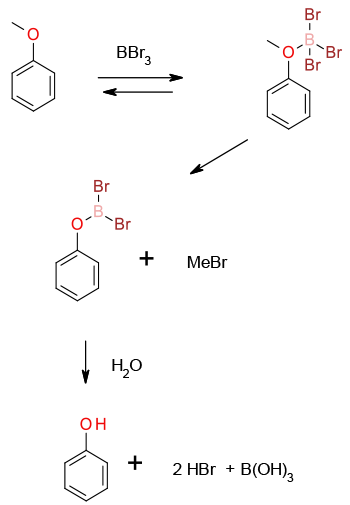 All these Lewis acids work by the same mechanism. Coordination to the ether oxygen increases the leaving ability of the latent phenoxide. The ether is then cleaved by an SN2 reaction generating alkyl halide and the boronate. Hydrolysis generates the required phenol, acid and boric acid.
All these Lewis acids work by the same mechanism. Coordination to the ether oxygen increases the leaving ability of the latent phenoxide. The ether is then cleaved by an SN2 reaction generating alkyl halide and the boronate. Hydrolysis generates the required phenol, acid and boric acid.
General comments
Main use is in the cleavage of methyl, ethyl and isopropyl groups but have been used for t-Bu ethers and esters. Typical reagents are boron halides (BCl3, BBr3) and aluminium halides (AlCl3). Other Lewis acids have been employed, but are less commonly used e.g. TiCl4, ScOTf3, YbOTf3. More complex boron reagents have been used to fine tune reactivity. These reagents are typically used to cleave aryl and heteroaryl ethers where the corresponding phenols are the desired products. The acid and salts generated can make work-up problematic and several of the references quoted quench with alcohols before adding water to minimise these issues. The use of halogenated solvents is commonplace, though usually through historic references rather than the availability of more green alternatives (the green solvent guide is a useful resource for alternatives).
As well as aryl alkyl ethers, Lewis acids can cleave alkyl-alkyl ethers, but the desired selectivity can be difficult to achieve.
Key references
J. Org. Chem., 1999, 9719 Cleavage of allyl ethers BCl3/n-Bu4NI
Org. Proc. Res. Dev., 2007, 731 BBr3 dealkylation
Syn. Comm. 1999, 29, 973 Dealkylation of alkyl and aryl ethers with AlCl3-NaI
Org. Proc. Res. Dev., 2003, 7, 379 BBr3 demethylation
Relevant scale up example
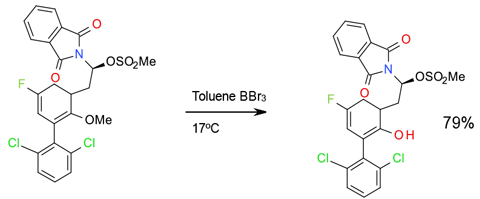
Experimental
25 Kg scale
Org. Res. Process Dev. 2010, 14, 1438
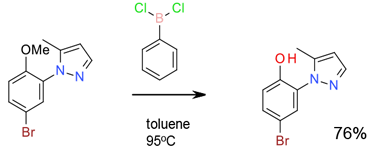
Experimental
700 gram scale
Org. Process Res. Dev. 2012, 16, 2031
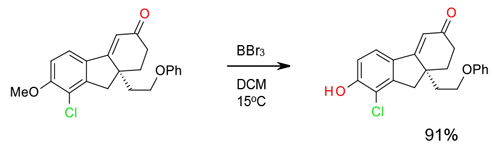
Experimental
7 Kg scale
Org. Process Res. Dev. 2008, 12, 723
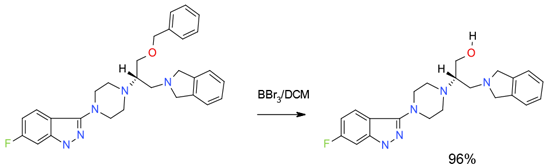
Experimental
4 Kg scale
Org. Process Res. Dev. 2003, 7, 521
Green Review
-
Atom efficiency (by-products Mwt)
Moderate atom efficiency, obviously worse for higher Mwt halides – generally not all halides are available for reaction. - Safety Concerns
No notable concerns – most Lewis acids are corrosive and react with water/ alcohols. The generation of lower Mwt alkyl halides may cause off-gassing. - Toxicity and environmental/aquatic impact
Alkyl halides will be generated as by-products. These are alkylating agents and will give rise to positive PGI alerts. There may be issues with discharging aqueous waste with high Al or B content. Hydrolysis of boron-based reagents will lead to boric acid which is a suspected reprotoxic mutagen and subject to potential restrictions through legislation (such as REACH) - Cost, availability & sustainable feedstocks
Reagents are generally readily available at reasonable cost. Neither Al or B are at risk of depletion, but this may not be the case with Lewis acids based on rare metals. - Sustainable implications
Best with Al or Ti –based Lewis acids. Boric acid is listed under REACH as a reagent (or in this case by-product) for reduction or preferably elimination.