Metal-Catalysed Ether Cleavages
Mechanism + Description
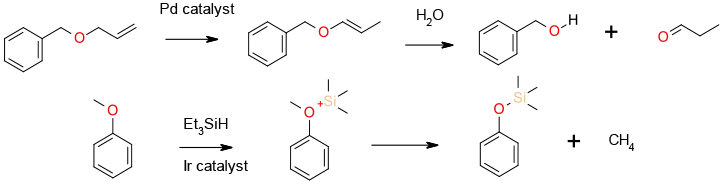
Pd & Rh complexes cleave allyl ethers by double bond migration to the vinyl ether followed by hydrolysis to the aldehyde. Ir/alkylsilanes reductively cleave the corresponding oxonium ion to generate a phenolate and alkane.
General comments
Pd complexes are used to catalyse the cleavage of allyl ethers and esters. A number of metal complexes are known to be active in the cleavage of alkyl aryl ether bonds to generate phenols. Ir pincer complexes in the presence of silanes react with anisoles to give phenoxysilanes which are hydrolysed to the phenols on work-up.
Key references
Cleavage of allyl ethers
J. Org. Chem., 1997, 62, 8932 – Pd(Ph3P)4, RSO2Na, CH2Cl2 (Example from process group,
Chem. Pharm. Bull., 1992, 40, 1718 – Pd(Ph3P)4, AcOH
J. Am. Chem. Soc., 2008, 130, 17509 Scope and Mechanism of the Iridium-Catalyzed Cleavage of Alkyl Ethers with Triethylsilane
Helv.Chim.Acta., 1985, 68, 618Rh mediated allyl ether cleavage
Relevant scale up example
None located