Metal Salts (halides)
Mechanism + Description
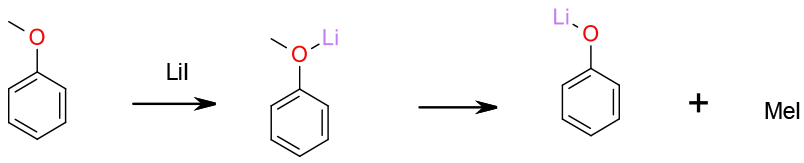
The alkyl group is cleaved by an SN2 mechanism to give metal phenoxides which are converted to phenols on quenching. The corresponding alkyl halide is generated as a by-product. The ether is activated by co-ordination of the metal cation.
General comments
Many metal salts and ionic liquids have been reported to cleave alkyl aryl ethers. These reagents all dealkyate via SN2 reaction of the anion liberating the phenol. Metals often promote the reaction by coordination to the ether oxygen and ionic liquids provide a medium which accelerates SN2 reactions. Oxophilic metals like Li , Zn and Mg are most common, and Iodide the most reactive anion, although bromides and chlorides are sometimes used in combination with catalytic amounts of iodide salts. This type of ether cleavage reaction has been reported to be promoted by the use of microwave heating and/or the use of ionic liquids.
Key references
Organic & Biomolecular Chemistry, 2009, 7, 5084 Selective demethylation and debenzylation of aryl ethers by magnesium iodide
Tetrahedron Letters, 2012, 53, 5376 Positional chemoselectivity in the Zn(II)-mediated removal of phenol protecting groups
Synthesis, 1989, 4, 287 Dealkylation of activated alkyl aryl ethers using lithium chloride in dimethylformamide
Relevant scale up example
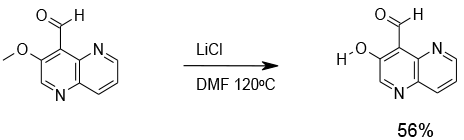
Experimental
10 Kg scale
Org. Process Res. Dev., 2012, 16, 788
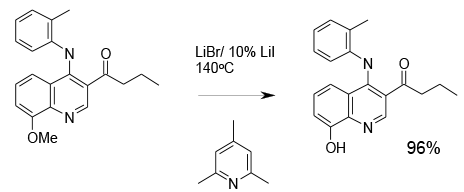
Experimental
Pilot scale
Org. Process Res. Dev., 1997, 1, 185
Green Review
-
Atom efficiency (by-products Mwt)
Dependent on salt and equivalents used. Lower Mwt metal with 1 equiv offers reasonable atom efficiency - Safety Concerns
No major issues identified. Low Mwt. alkyl halides may off gas from reaction and require abatement. - Toxicity and environmental/aquatic impact
Major concerns here would relate more to solvents used than metals. Li and Iodide are of concern in fresh water ecosystems. Alkyl halides will be generated as by-products. These are alkylating agents and will give rise to positive PGI alerts. - Cost, availability & sustainable feedstocks
Generally readily available and cheap – although there is a premium for anhydrous metal salts. Cl and Br are preferred anions over iodide. - Sustainable implications
Many of these dealkylation reactions with metal salts are conducted in dipolar aprotic solvents, which should be replaced if possible. Use of Iodine could give rise to problems with the incineration of iodide containing waste streams. Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion, although it is possible to recover iodide from waste materials.
Li, Zn and Magnesium are rated at moderate risk of depletion.