DDQ/CAN Oxidation
Mechanism + Description
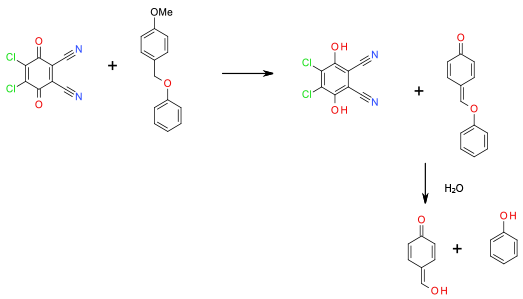
Oxidation to the corresponding quinone methide followed by hydrolysis. Thought to be singlet electron Mediated (see third ref below).
General comments
Oxidative cleavage is of limited use – mainly applied in the cleavage of p-methoxybenzyl and related ethers, but can be useful where differentiation between benzyl ethers is required. DDQ and ceric ammonium nitrate are the most commonly employed oxidants.
Key references
J. Chem . Soc. Perkin Trans. 1, 1984, 2371 Ceric ammonium nitrate (CAN), (NH4)2Ce(NO3)6
Tetrahedron, 1986, 3021 Use of DDQ
P-methoxybenzyl ethers as protecting groups – useful discussion of oxidative mechanisms and applicability.
Relevant scale up example
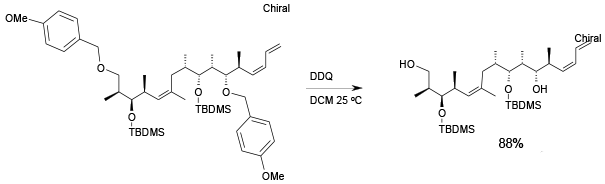
Experimental
1.5 kg scale
Org. Process Res. Dev., 2004, 8, 113
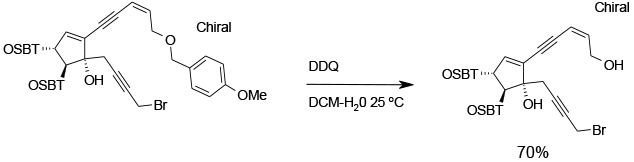
Experimental
Gram scale
Org. Process Res. Dev., 2009, 13, 1111
Green Review
-
Atom efficiency (by-products Mwt)
Both reagents show moderate to poor atom economy. Ce(IV) is a one electron oxidant so at least 2 equivalents of (NH4)2Ce(NO3)6 are required. DDQ generates the corresponding 1,4 hydroquinone as a by-product (230). - Safety Concerns
DDQ can decompose when heated to give HCN. - Toxicity and environmental/aquatic impact
Cerium is toxic to aquatic organisms. DDQ can decompose in water to liberate HCN. - Cost, availability & sustainable feedstocks
Reagents are reasonably available, but not the cheapest available for deprotection. - Sustainable implications
Ce is at high risk of depletion. DDQ reactions are routinely undertaken in chlorinated solvent.