Thiols/Methionine
Mechanism + Description
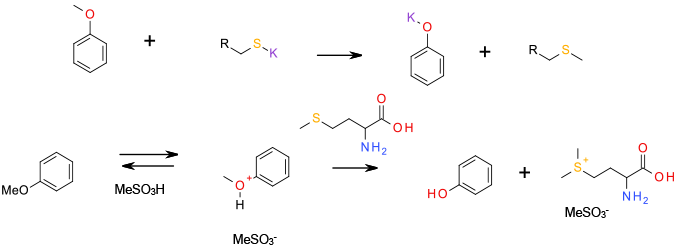
A standard SN2 reaction with a thio-anion generating a phenoxide anion and a thio ether, or sulfonium species in the case of methionine, the reaction is run in strong acid to protonate the ether and increase the electrophilicity of the leaving alkyl group.
General comments
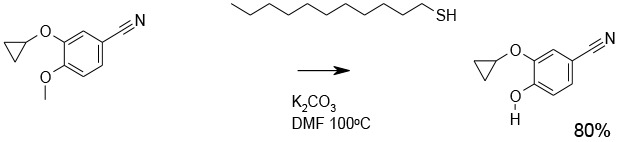
A wide range of thiols have been employed as nucleophiles to cleave aryl methyl ethers via an SN2 reaction. often high molecular weight/ low volatility thiols are employed to reduce odour issues. These are usually applied as thiolate salts either added pre-formed or made in situ. The choice would probably come down to a screening exercise, but generally solvents used are dipolar aprotic to enhance the SN2 reaction. A number of published methods use DMF or HMPA. These solvents are probably best avoided if alternatives can be located. Sulfolane, tetramethylurea and DMI are good alternatives for dealkylation with thiols. Successful cleavage of alkyl aryl ethers often critically depends on correct choice of solvent and base (J. Org. Chem., 2002, 67, 6406).
Some thiolate dealkylations have been reported to be accelerated by microwave heating.
Methionine is a relatively new reagent for scale-up, used for the cleavage of alkyl aryl ethers to phenols. The resulting alkylated methionine is non-volatile and non-genotoxic (a vitamin). The reaction is often carried out in heated methane sulphonic acid, which on its own can cleave alkyl aryl ethers, so the presence of methyl mesylate as a by-product cannot be excluded. The stereochemistry of the methionine used is not relevant to this process.
Key references
J. Org. Chem., 1977, 42, 1228 Regioselective O-demethylation
Archives of Pharm. Research, 2008, 31, 305 Practical demethylation of aryl methyl ethers using an odorless thiol reagent
Heterocycles, 2001, 54, 989 Sodium ethanethiolate / dimethylformamide / 3.5 h / 130 °C
J. Org. Chem., 2006, 71, 7103 2-(Diethylamino)ethanethiol, a New Reagent for the Odorless Deprotection of Aromatic Methyl Ethers
J. Org. Chem., 1987, 52, 5143 Use of methionine
J. Chem. Soc., Perkin Trans. 1, 1977, 2288 – Regioselective cleavage of aromatic methyl ethers by methanesulphonic acid in the presence of methionine
Relevant scale up example
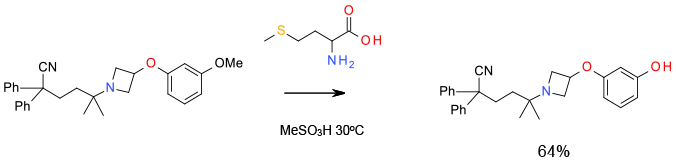
Experimental
2.5 kg scale
Org. Process Res. Dev., 2012, 16, 195
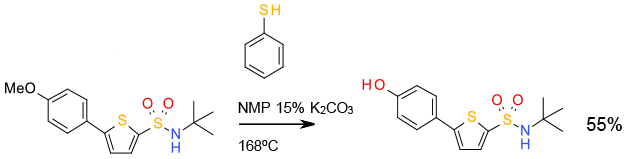
Org. Process Res. Dev., 2009, 13, 751
Experimental
30 kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Depends on the thiol used – low Mwt. preferred, but these give issues with odor. - Safety Concerns
The safety concerns outlined below can usually be overcome for scale-up, though do mean that low Mwt thiols are less preferential on scale. Many thiols and to a lesser extent thio ether by-products have odour issues – many have very low odour thresholds and can be very persistent. Low Mwt thiols are toxic and highly flammable. - Toxicity and environmental/aquatic impact
No major issues apart from the toxicity of low Mwt thiols. Heating methyl aryl ethers with methane sulfonic acid may lead to formation of methyl mesylate (PGI). - Cost, availability & sustainable feedstocks
Most thiols are readily available at reasonable cost – most are derived from petrochemical sources. Methionine is a natural product. - Sustainable implications
If the thiol dealkylation is performed as a metal salts, they are usually conducted in dipolar aprotic solvents which should be replaced if possible. Methanesulfonic acid has quite a high LCI.