Trimethylsilyl Iodide (TMSI)
Mechanism + Description
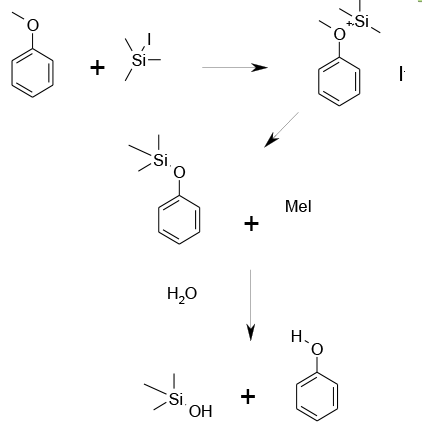
TMS Iodide works by a similar mechanism to Lewis acids. Coordination to the ether oxygen increases the leaving ability of the latent phenoxide. The ether is then cleaved by an SN2 reaction generating alkyl halide and the silylate. Hydrolysis generates the required phenol, acid and trimethylsiloxide, which will form TMS2O.
General comments
TMSI is often used to cleave aryl alkyl ethers to generate phenols, although can be used to cleave dialkyl ethers and deprotect t-Butyl esters. Due to cost and instability of the reagent, it is often generated in situ from TMSCl and NaI.
Key references
Tetrahedron,1982, 38, 2225 Iodotrimethylsilane —a versatile synthetic reagent
J. Org. Chem., 1977, 42, 3761 Quantitative dealkylation of alkyl ethers via treatment with trimethylsilyl iodide
J. Chem. Soc. Chem. Commun., 1978, 20, 874 Novel method for dealkylation of esters, ethers, and acetals by chlorotrimethylsilane-sodium iodide
Relevant scale up example
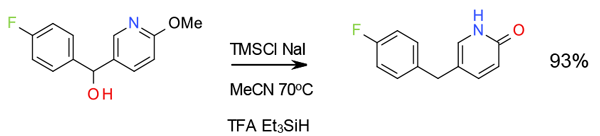
Org. Process Res. Dev., 2007, 11, 899
Experimental
1 Kg scale
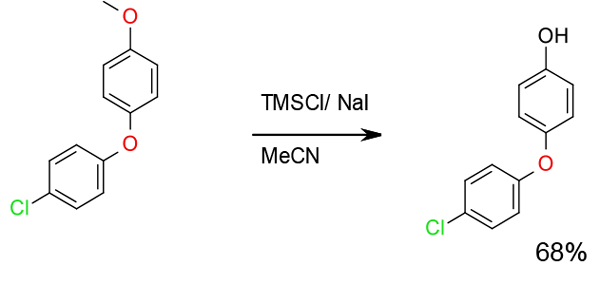
Org. Process Res. Dev., 2009, 13, 1177
Experimental
10 Kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Poor atom economy – dealkylation generates TMS2O (162) after quench and the corresponding alkyl iodide. - Safety Concerns
None apparent. TMSI is corrosive and reacts with water. TMSI may be incompatible with certain solvents, e.g. ethers. - Toxicity and environmental/aquatic impact
Organoiodine compounds, especially hydrophobic materials, present a moderate hazard to the aquatic environment and may bioaccumulate. TMS2O will not biodegrade. Alkyl iodide by-products may present PGI issues, and volatile organoiodides like MeI may need off gas control. Possible issues with any incineration of waste solvents. - Cost, availability & sustainable feedstocks
Generally a high cost reagent for organic synthesis on large scale – preparation from TMSCl and NaI greatly reduces cost and reduces stability on storage concerns. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. High LCI reagent, although it is possible to recover iodide from waste materials.