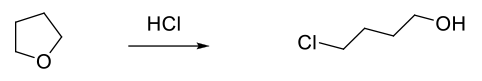Specific solvent issues with BOC deprotection
Traditional reagents to remove BOC are TFA in dichloromethane and HCl in 1,4- dioxan, and these mixtures are still widely used in Medicinal Chemistry. Dichloromethane is harmful to the aquatic environment and a suspect carcinogen.1,4-dioxan is a suspect carcinogen. In almost all cases, these solvents can be replaced by greener materials from a range of solvent classes – ketones, esters, ethers and aromatic hydrocarbons – although the later are best substituted.
Since the amine product after BOC removal is often isolated and purified as the conjugate salt of the acid used for deprotection, solvent choice is often governed by an acceptable reaction rate and good crystallisation of the amine salt.
Ethers like THF and 2-MeTHF are increasing seen as replacements for 1,4-dioxan. Some caution needs to be exercised with ethers since strong acids like HCl (also Lewis acids) can cleave ethers to chloro alcohols. These materials can hamper product isolation and are also classed as PGI’s.