Bases
Mechanism + Description
Mechanism depends on substrate. With a primary amine deprotonation followed by isocyanate formation and hydrolysis to product -A. Where a good leaving group can be formed, an alternative mechanism is addition of OH- or RO- followed by loss of the amine anion-B. A third proposed mechanism is via a β type elimination type fragmentation following deprotonation of a methyl group.
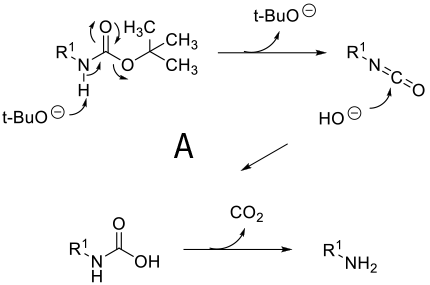
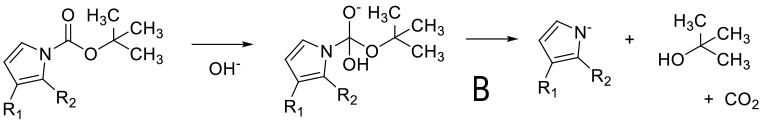
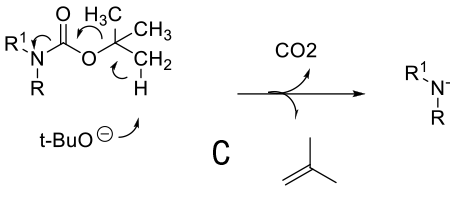
General comments
Although not as widely used as acid deprotection, some BOC amines can be deprotected under basic conditions. This chemistry is limited mainly to primary amines and is promoted by R groups that are electron-withdrawing facilitating easier deprotonation & a highly stabilized anion/leaving group can be formed e.g. heterocycle leaving group or imide equivalents like RN(BOC)2, R(NBOC)SO2R. Typical heterocycles are indoles, azaindoles, indazoles, pyrazole, indolinone, quinolinone, and oxazolone. Bases used include t-BuONa, NaOMe, Cs2CO3, Na2CO3. Base deprotection may be chosen over acid when other acid sensitive functionality is present, or selective deprotection of multiply BOC’d compounds.
Key references
Tetrahedron Lett. 2006, 47, 8575-8577 A mild and selective method for the N-Bocdeprotection by sodium carbonate
Tetrahedron Lett. 2004, 45, 905-906. Deprotection of a primary Boc group under basic conditions
Synth. Commun. 2007, 37, 281-287 Simple and Selective Removal of the t‐Butyloxycarbonyl (Boc) Protecting Group on Indoles, Pyrroles, Indazoles, and Carbolines
ARKIVOC 2005 (xiv) 20-28 Efficient and selective cleavage of the tert-butoxycarbonyl (Boc)
Relevant scale up example
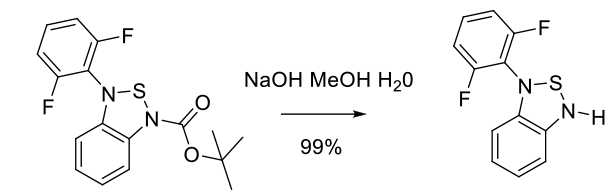
Experimental
25 kg scale
Org. Process Res. Dev. 2010, 14, 868–877
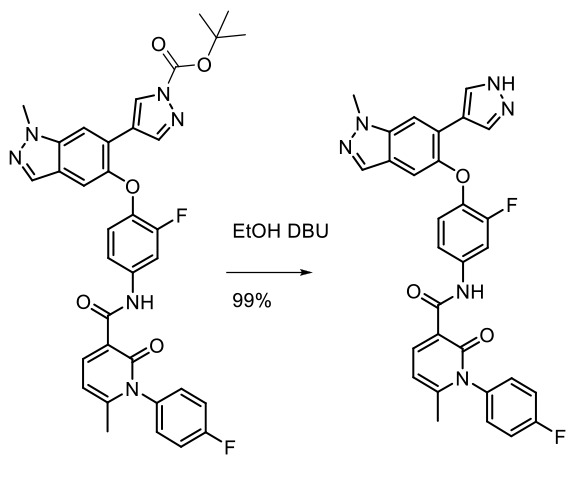
Experimental 25 Kg scale
Org. Process Res. Dev. 2014, 18, 501−510
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficiency is high for simple inorganic bases, the product results as a salt. Higher alkoxides and organic reagents like DBU give poorer atom economy, and should be used catalytically if possible.
Atom Efficiency (By-Products Mwt < 200 g/mole):- NaOtBu By-Products (assuming 1 equiv of base) = CO2 (44.0 g/mole) + tert-butanol (74.1 g/mol) + NaOtBu (96.1 g/mole) = 214.2 g/mole
- NaOMe By-Products (assuming 1 equiv of base) = CO2 (44.0 g/mole) + methyl tert-butyl ether (88.2 g/mol, assumed by-product) + NaOMe (54.0 g/mole) = 186.2 g/mole
- Na2CO3 By-Products (assuming 0.5 equiv of base) = CO2 (44.0 g/mole) + water (18.0 g/mole) + NaOtBu (96.1 g/mole) = 158.1 g/mole
- Safety Concerns
No apparent major operational issues – Glass corrosion can be an issue with solutions >1 M above 40 °C .Care needs to be taken as caustic solutions are corrosive. Strongly basic solutions maybe incompatible with chlorinated solvents. Alkoxide salts can be flammable and air reactive. - Toxicity and environmental/aquatic impact
Inorganics – generally minimal issues once neutralized and diluted. Lithium containing aqueous effluents will be toxic and difficult to treat – Lithium is very toxic to freshwater ecosystems. Na and K are preferred salts. No environmental issues with lower alcohols from alkoxides, although t-BuOH is difficult to biodegrade. Higher Mwt amines maybe ecotoxic and accumulative. - Cost, availability & sustainable feedstocks
Generally cheap and readily available, linear low Mwt alkoxides can be made from biorenewables. - Sustainable implications
Very sustainable if aqueous waste can be directly bio-treated. Lithium is currently rated as medium to high risk of depletion.