Use of Base Metals in Buchwald-Hartwig Coupling
Mechanism + Description
Use of base metal complexes to replace Pd – mechanism as for Pd coupling
General comments
In order to replace the high impact metal, Pd, a number of catalyst systems using base metals like Nickel have been reported for B-H amination. Recent advances in the Ullman reaction using Cu in the presence of various ligand has made this metal an attractive alternative to Pd catalysed B-H amination.
Key references
Relevant scale up examples with Scheme
None located
Green Review
-
Atom efficiency (by-products Mwt)
With optimized metal and ligand stoichiometry, catalytic metals and ligands have negligible contribution to the atom/mass intensity. Bi-products are inorganic salts/amine salts/alcohols. In terms of leaving groups Cl- Safety Concerns
No major concerns around scaling B-H aminations. Lower mol wt alkylphosphines can be highly flammable.- Toxicity and environmental/aquatic impact
Main concern is around loss of precious metal/ heavy metal catalysts into waste streams. Most precious and heavy metal levels are tightly regulated. The same applies to potential carry through into the API. See next slide for more details. Some Ni salts are sensitizers and carcinogens and listed on the EU SVHC list – this is of less concern for metallic hydrogenation catalysts. Hydrophobic, high mol wt phosphines can be persistent and bioaccumulative and should not be discharged into aqueous waste streams.
Simple bases like Na/K hydroxides, carbonates, bicarbonates and phosphate are preferred over organic amines and alkoxides. Local regulations may limit concentrations of phosphate that can be discharged in aqueous waste Cs2CO3 is widely used in Suzuki reactions. This should be substituted if possible.- Cost, availability & sustainable feedstocks
With high catalytic efficiency, this methodology can be an economical way to access aryl/heteroaryl amine compounds. All metal catalysts have a high LCI from mining and refining and would need to be recovered and recycled.- Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with efficient recovery and recycle. Pd is most commonly used precious metal for hydrogenation and this is rated at high risk of depletion. No concern for abundant base metals like Ni, Cu etc. If aryl iodides are used, incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. High LCA reagent, although it is possible to recover iodide from waste materials. - Safety Concerns
Updated ICH Guidelines for Metals in API’s
www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step2b.pdf
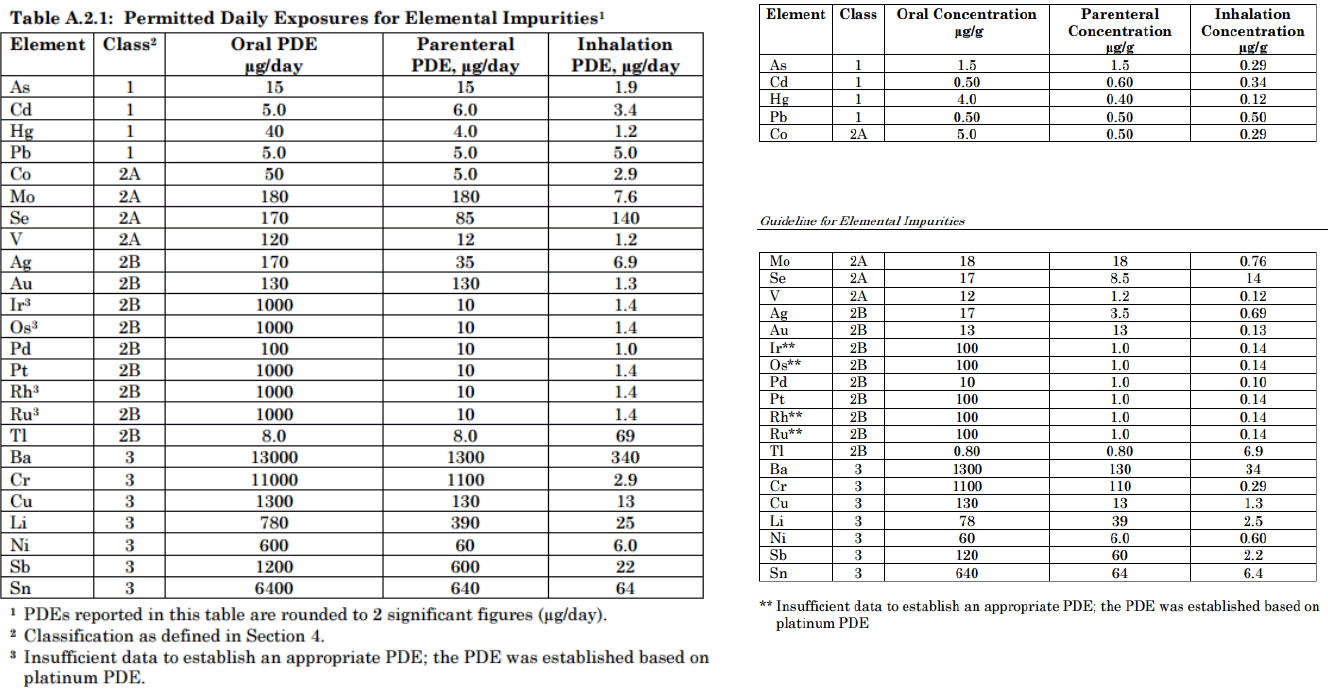
PDE = Permitted daily exposure