Synthesis of Primary Amines / Hydrazines via Buchwald-Hartwig Amination
Mechanism + Description
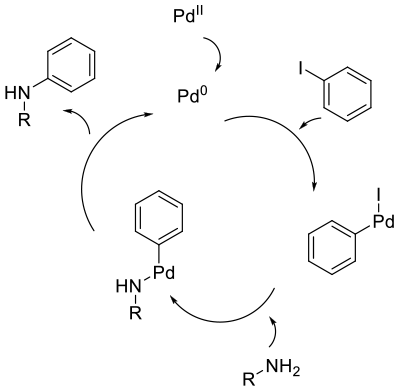
Mechanism as for Pd catalysed B-H amination for secondary and tertiary amines.
General comments
Primary amines can be constructed using B-H amination methodology. Initially, ammonia equivalents like HN(TMS)2 or benzophenone imine were used followed by deprotection to reveal the primary amine. Catalyst systems have now been identified that work with NH3 directly thus making this transformation more mass efficient. Likewise, aryl/heteroaryl hydrazines can be prepared from benzophenone hydrazone, or with certain ligands, N2H4 directly.
Key references
J. Org. Chem., 2008, 73, 8880-8892 Use of HN(TMS)2 as an NH3synthon in Buchwald-Hartwig amination
Chem. Soc. Rev., 2010, 39, 4130-4145 Direct amination of aryl halides with ammonia
Relevant scale up examples with Scheme
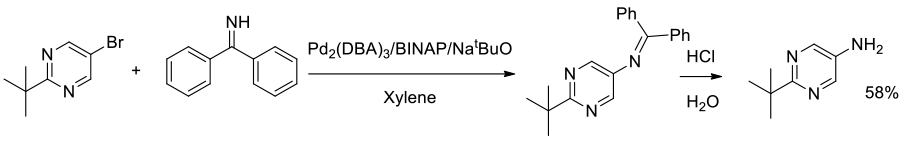
Org. Process Res. Dev., 2006, 10, 70-77
Experimental
7 kg scale
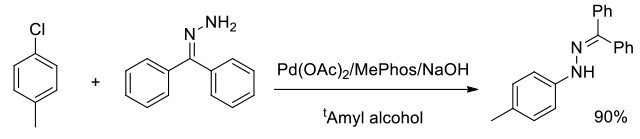
Org. Process Res. Dev., 2014, 18 (12), 1752–1758
Experimental
1 kg scale
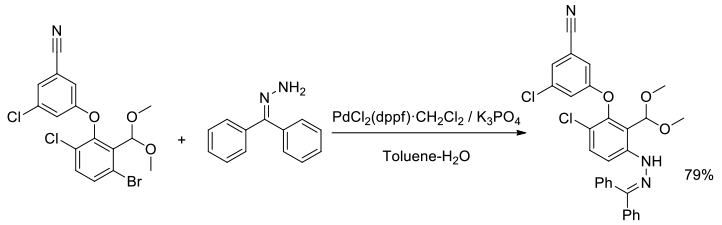
Org. Process Res. Dev., 2008, 12, 512–521
Experimental
8 kg scale