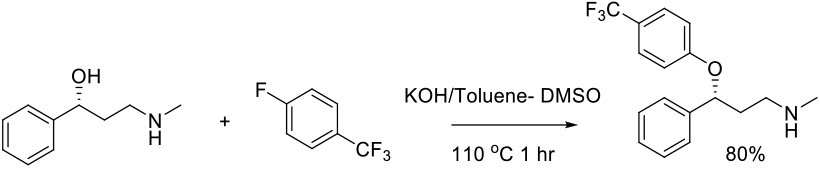
SNAr Reaction in S-based Solvents
Mechanism + Description
As per N-based dipolar aprotic solvents.
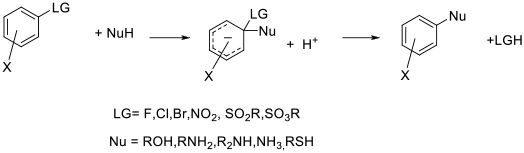
General comments
Apart from the N-based dipolar aprotic solvents, DMSO is often employed as a solvent for SNAr chemistry. DMSO has a good toxicity and environmental profile, but does present some thermal hazards at higher temperature in the presence of electrophiles and/or strong bases. Recent work has demonstrated that some Buchwald-Hartwig couplings can be performed in DMSO as standard SNAr reactions, negating the need for Pd catalysts.
Sulfolane has been proposed as a solvent with similar properties to DMSO, but is much less widely used presumably due to its high melting point of 28°C. Sulfolane was assumed to have similar toxicity profile to DMSO, but recent data suggests there may be concerns with his material–see solvents section.
Piperylene sulfone and butadiene sulfone have been proposed as alternatives to DMSO and sulfolane. These materials is are formed from the [4+2] cycloaddition of SO2 and the cis diene. The claimed attraction is that volume inefficient work-ups normally associated with dipolar aprotic solvents can be avoided by heating the solvent to ~100°C which reverses the cycloaddition to give two gaseous products which can be separated then recombined. Safety issues around handling gaseous butadiene or piperylene in standard chemical plants would detract from substituting for commonly used solvents.
Key references
Org. Process Res. Dev. 2012, 16, 1273−1278 Sulfolane: A Versatile Dipolar Aprotic Solvent
Tetrahedron 2018, 74, 303-307 Base-promoted nucleophilic fluoroarenes substitution of CF bonds
J. Org. Chem. 2015, 80, 4213−4220 An SNAr Approach to Sterically Hindered ortho-Alkoxybenzaldehydes
Chem. Comm. (2007) 14:1427–1429 Piperylene sulfone: a labile and recyclable DMSO substitute
Relevant scale up examples in DMSO with Scheme
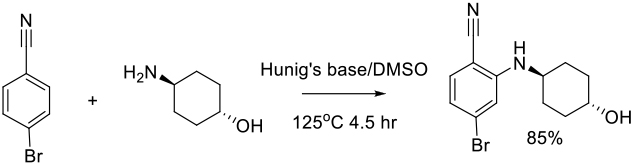
Org. Process Res. Dev. 2012, 16, 1787−1793

Org. Process Res. Dev. 2010, 14, 168–173
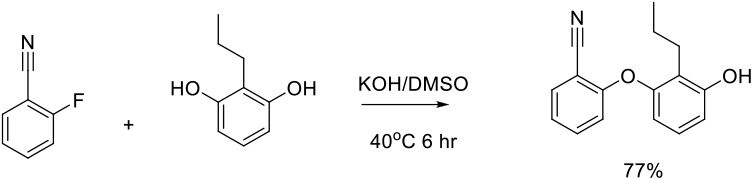
Org. Process Res. Dev. 2009, 13, 268–275