Hydrogenation with Frustrated Lewis Pairs (FLPs)
Mechanism + Description
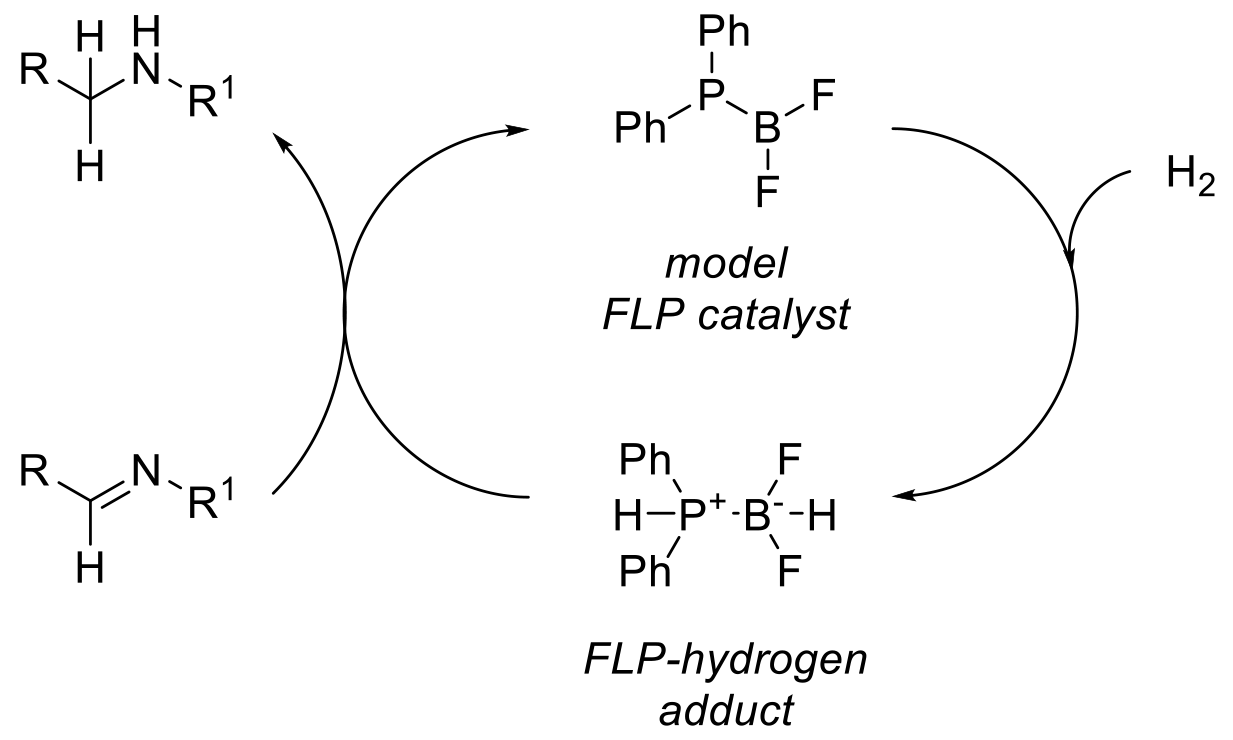
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acidic atom and a Lewis basic atom that cannot combine to form a classical donor-acceptor adduct due to steric hindrance. The catalytic activity of FLP compounds to activate hydrogen is exemplified by the following reaction.
PCy3 + B(C6F5)3 + H2 → [HPCy3]+[HB(C6F5)3]−
General comments
The activation and transfer of hydrogen by FLP catalysts is currently an area of intense research interest, and offers the ability to run metal-free catalytic hydrogenations since the FLP hydrogen adduct will directly transfer H2 to a range of unsaturated molecules traditionally reduced using heterogeneous metal catalysts/H2 or hydride reagents. Due to the more ionic nature of the FLP-H2 adduct, such FLP catalytic hydrogenations have been targeted at more polar double bonds such as imines and enamines, but examples exist of a wider range of substrates such as olefins, alkynes, and aromatic systems.
Use on scale is somewhat limited by both current commercial availability of FLP catalysts and high loadings due to low reaction rate and in situ deactivation, though there is significant research activity in developing more robust and active FLP catalysts for a range of chemistries.
Key references
Relevant scale up examples
No scale-up examples found.