Deprotection by Lewis Acids
Mechanism + Description
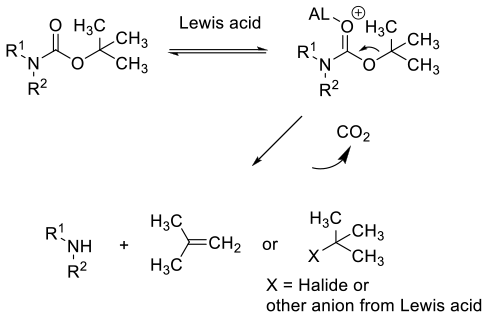
Lewis acid-catalyzed deprotection mirrors acid deprotection – coordination of the Lewis acid rather than protonation, followed by fragmentation to the amine, CO2 and a transient t-Bu+ cation which can form isobutylene or react with any nucleophilic species present. Isobutylene is off-gassed, or under acidic conditions can oligomerise. Typically the Lewis acid needs to be used in greater than stoichiometric amounts.
General comments
While many different Lewis acids have been reported to deprotect BOC amines, they are seldom used on scale, with acids being the reagents of choice. There may be some advantage when multiple acid sensitive groups are present in the substrate. It should be borne in mind that under protic conditions – water/alcohols or with adventitious water, many Lewis acids and other reagents (Me3SiCl) claimed to remove BOC groups will hydrolyse to give protic acids, which are the active catalyst in the deprotection. Another limitation is that many weaker Lewis acids under mild conditions will only remove BOC groups from imide type compounds – N(SO2R)BOC.
Key references
RSC Adv., 2015,5, 6647-6651 A practical, catalytic and selective deprotection of a Boc group in N,N′-diprotected amines using iron(III)-catalysis
Chem. Commun., 1996, 2509-2510 Extremely mild reagent (SnCl4) for Bocdeprotection applicable to the synthesis of peptides with thioamide linkages
Synthetic commun. 1989, 19, 3139-3142 Selective Removal of the tert-Butoxycarbonyl Group from Secondary Amines: ZnBr2 as the Deprotecting Reagent
Synthesis 1999(1): 66-68 Lewis Acid-Mediated (AlCl3) Selective Removal of N-tert-Butoxycarbonyl Protective Group (t-Boc)
Relevant scale up example
None located.