Thermal Methods
Mechanism + Description
Many BOC amines can be deprotected simply by heating at high temperature without any added catalyst. The mechanism is probably via fragmentation and formation of the amine via the carbamic acid, isobutylene and CO2.
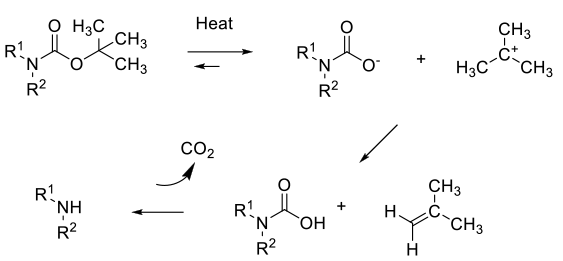
General comments
A potentially green route to remove BOC groups since no catalyst or acid is added. The drawback is that temperatures of around 150° C or higher are needed for a practically useful rate. Some substrates might lose the BOC group at ~100° C
But reaction times are in the order of 2-3 days. High temperatures may cause side reactions like elimination and racemisation of chiral substrates. Hot, super heated or near critical water are good solvents for thermal BOC deprotection, but may not readily available in standard chemical processing labs/plants, requiring pressurized reactors or flow chemistry.
- Green Chemistry Overview:
- Atom Efficiency (By-Products Mwt < 200 g/mole):
- CO2 (44.0 g/mole) + isobutylene (56.0 g/mol) = 100 g/mole
- Toxicity and Limited Process Safety Hazards:
- Low toxicity
- High pressure if above 100 °C (process safety hazard)
- High temperature (process safety hazard)
- Benign Environmental Impact:
- Low environmental impact
- Atom Efficiency (By-Products Mwt < 200 g/mole):
Key references
Tetrahedron Lett. 2009, 50, 1438–1440 Catalyst-free water-mediated N-Bocdeprotection
Chem. Commun., 2009, 5144–5146 Boiling water-catalyzed neutral and selective N-Bocdeprotection
International Journal of Chemistry; Vol. 4, No. 3; 2012 73-79 A Simple and Efficient Green Method for the Deprotection of N-Boc
Green Chem., 2014,16, 2147-2155 Water at elevated temperatures (WET): reactant
Synthetic Commun. 1996, 26, 3999-4004 Thermal Removal of Boc-Protecting Groups During Preparation of Open-Chain Polyamines
Relevant scale up example
None located.