Preparation of sec and tert amines by Buchwald-Hartwig Amination
Mechanism + Description
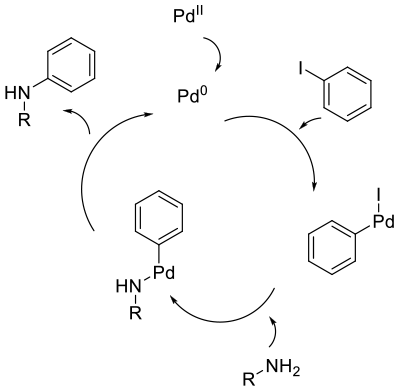
The B-H amination follows the usual pathway of oxidative insertion of a Pd(0) catalyst into an aryl halide/sulfonate followed by coordination of the amine followed by reductive elimination to give the product. If a Pd(2+) salt/complex is added, pre-reduction to the active Pd(0) catalyst is needed. The role of the base is to deprotonate the amine prior to, or after coordination to the Pd catalyst.
General comments
Typically the reagents are mixed with a Pd complex or a mixture of a simple Pd salt/complex and added ligands to generate the active catalyst in situ. The Pd can be added as Pd(2+) –usually Pd(OAc)2– or Pd (0) –usually Pdx(DBA)y. Traditional ligands for the B-H amination reaction are are Ph3P and (o-Tolyl)3P, although since first published the B-H amination has been reported with a wide range of other ligands – principally phosphines and stabilised carbenes – which have been developed to fine tune catalytic activity and allow reaction with traditionally difficult or unreactive coupling partners like aryl chlorides and very electron rich aryl / hetero-aryl bromides. Correct choice of ligand can also minimise reductive dehalogenation of the aryl halide coupling partner. Phosphine ligands cover mono and bidentate as well as very sterically hindered ligands. In many cases, phosphine ligands developed for other transformations like chiral hydrogenation are used in B-H amination reactions, and occasionally chiral ligands are used when there is no chiral demand in the product. Bases are typically inorganics like NaOH, Na/K/Cs2CO3, Na/KHCO3 and KXHYPO4 or alkoxides – Na/K t-BuO. Occasionally organic amine bases are employed.
Key references
Chem Sci., 2011; 2(1): 27–50 DialkylbiarylPhosphines in Pd-Catalyzed Amination: A User’s Guide
J. Org. Chem., 2014, 79, 11961–11969 Role of the Base in Buchwald–Hartwig Amination
Org. Process Res. Dev., 2014, 18, 180−190 Carbon−Heteroatom Coupling Using Pd-PEPPSI Complexes
Relevant scale up examples with Scheme
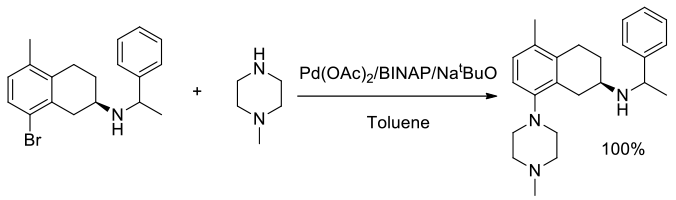
Org. Process Res. Dev., 2008, 12, 512–521
Experimental
Multi- kg scale
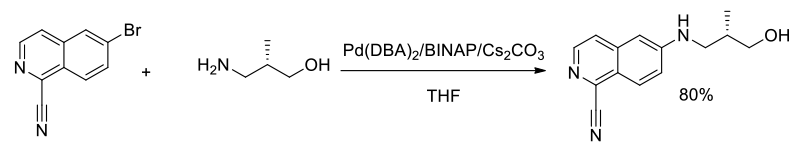
Org. Process Res. Dev., 2014, 18 (12), 1752–1758
Experimental
2 kg scale
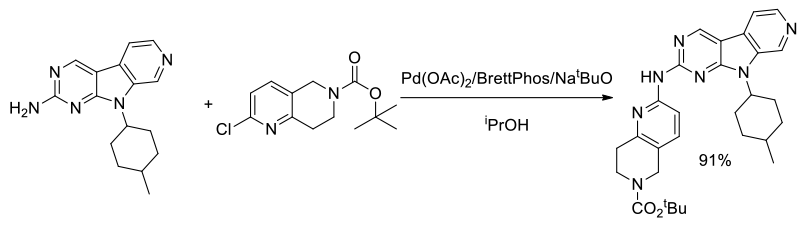
Org. Process Res. Dev., 2015, 19 (3), 476–485
Experimental
8 kg scale
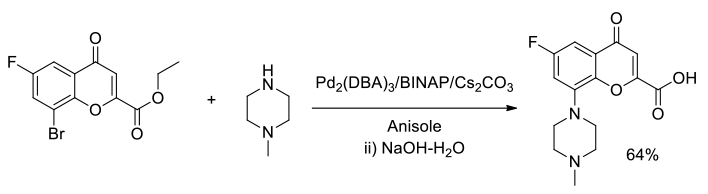
Org. Process Res. Dev., 2004, 8, 925-930
Experimental
40 kg scale
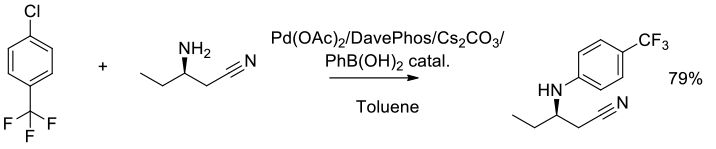
Org. Process Res. Dev., 2006, 10, 472-480
Experimental
4 kg scale
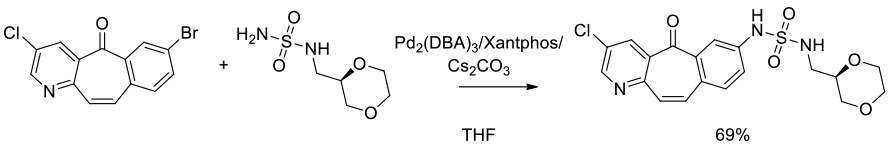
Org. Process Res. Dev., 2010, 14, 849–858
Experimental
3.5 kg scale
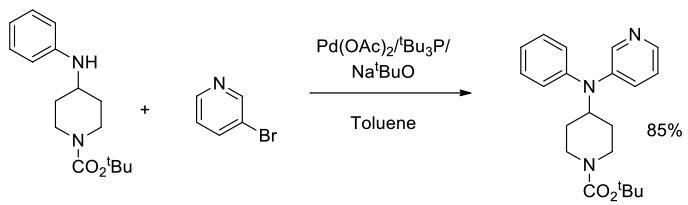
Org. Process Res. Dev., 2014, 18 (12), 1752–1758
Experimental
250 g scale
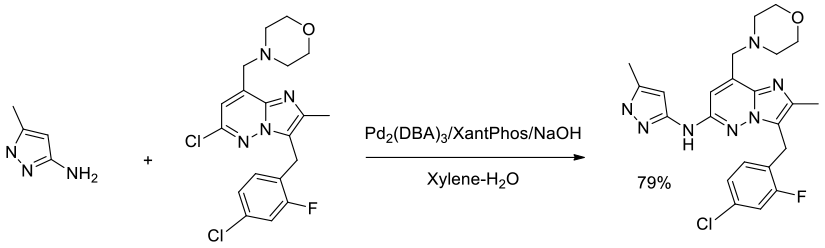
Org. Process Res. Dev., 2012, 16, 70–81
Experimental
83 kg scale
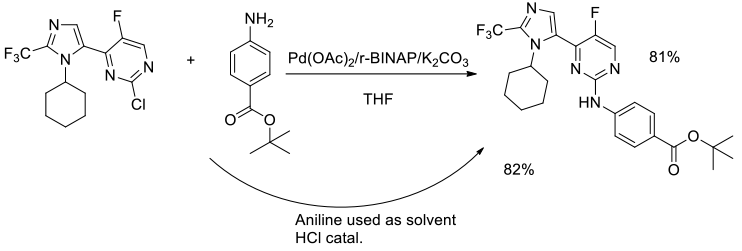
Org. Process Res. Dev., 2013, 17, 672−678
Experimental
50 g scale