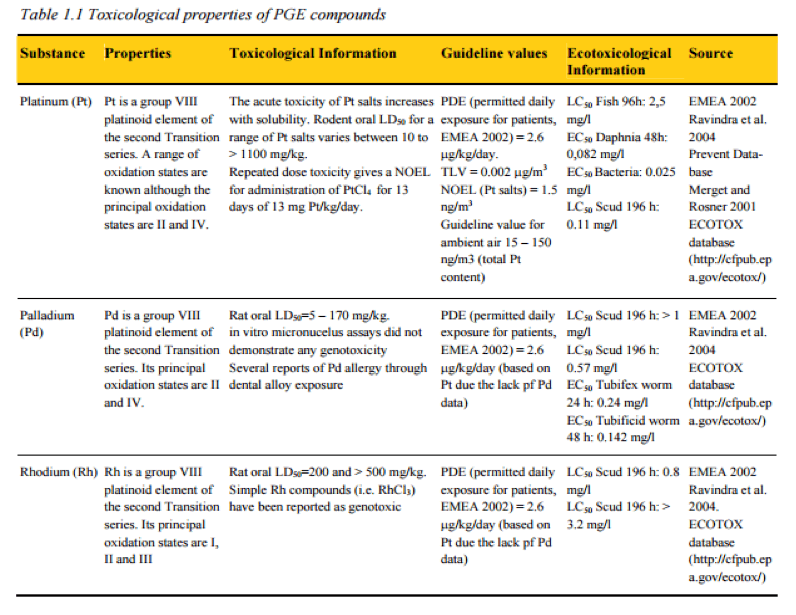
Tox Properties of Precious Metals
http://www3.ivl.se/miljo/projekt/dvss/pdf/katalysatormetaller2007.pdf
Most metal removal/scavenging methods fall into three categories:
- Use of soluble reagents to maintain metals in solution while the product is isolated by crystallization or precipitation (crystallisation preferred).
- Addition of reagents to precipitate the metal keeping the product in solution for later isolation. Precipitation may be as an insoluble metal complex or as elemental metal.
- The use of scavenger solids to bind metal particles or metal complexes. These can be fairly non-specific absorbents like carbons or more specific functionalised polymers/resins designed to sequester metals. The use of hybrid methodologies using both soluble reagents and solid scavengers is reasonably common.