Biocatalysis
Mechanism + Description
Depends on enzyme class. Amino acid dehydrogenases (AAD) react by reducing imines formed from α-ketoacids and NH3, and imine reductases reduce classical imines – usually cyclic in nature, and formed in situ. Both enzyme classes use a co-factor, NAD(P)H to deliver hydride. A co-factor recycling mechanism is therefore needed. The mechanism of ω-transaminase is not strictly reductive amination. These enzymes work by transfer of an amino group from a donor amine to the substrate ketone/aldehyde, so there is no redox process, but the reaction equilibrium needs to be driven in the appropriate direction.
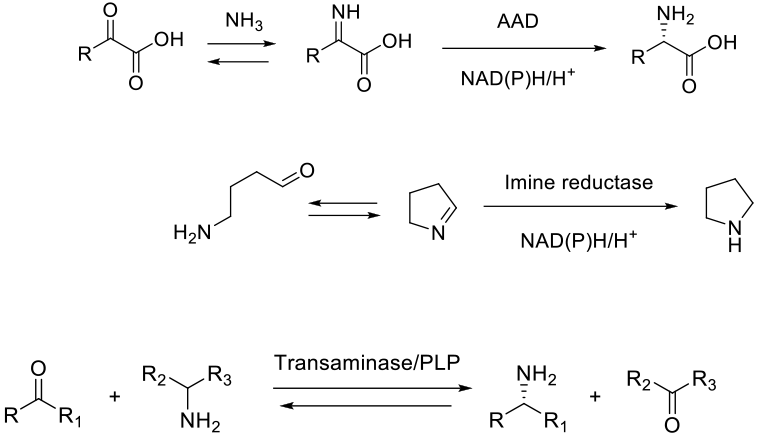
General comments
Biocatalytic methods generally work under mild conditions near to pH 7 and ambient temperature. The high degree of chiral selectivity makes biocatalytic methods attractive for the synthesis of chiral amines from aldehydes/ketones. For a given amine product, a number of bio routes potentially exist. The choice normally comes down to substrate/enzyme availability and achieving the desired purity/throughput.
Key references
ACS Catal., 2012, 2, 993−1001 ω-Transaminases for the Production of Optically Pure Amines and
ChemBioChem., 2014, 15, 2201 – 2204 Enzyme Toolbox: Novel Enantiocomplementary Imine
Relevant scale up examples
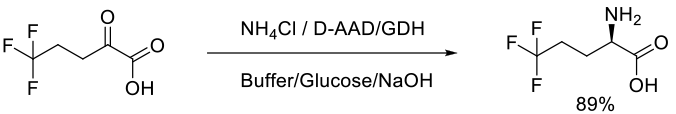
Org. Process Res. Dev., 2013, 17, 693−700
Experimental
50 g scale
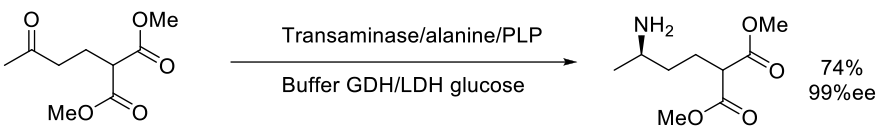
Org. Process Res. Dev.. 2013, 17, 61–68
Experimental
4.5 kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Excellent with good catalytic activity – impact of amine/ammonia synthons need consideration as does any co-factor recycling system to the mass intensity of the reaction. - Safety Concerns
Generally biocatalytic methods are free of thermal events and can be managed in standard equipment. - Toxicity and environmental/aquatic impact
The biocatalysts are commonly biodegradable, and pose minimal environmental hazards. Some of the proteins, especially dusty solids can be sensitizing (R42) so appropriate handling should be employed. For use in c-GMP manufacture of API, enzymes from mammalian sources or those fermented using mammalian products should be avoided. Older work using whole cells and biphasic systems often use dialkyl phthalates as the organic phase. These materials are persistent and endocrine disruptors hence should be avoided. - Cost, availability & sustainable feedstocks
Many enzymes are now becoming commercially available in bulk, and many CRO’s can offer biocatalysis and enzyme development services. In most cases, at the pilot stage, a biocatalysed reaction will cost more than a chemical alternative (fermentation / enzyme supply is very sensitive to economies of scale), but at full scale most biocatalysed processes are greener and cheaper than chemical alternatives. - Sustainable implications
Enzymes are produced from renewable materials, and are fully biodegradable back to innocuous natural products (amino acids). The use of modern molecular biology and fermentation technology has greatly reduced the LCI of enzyme manufacture. Maximum sustainability benefits are usually obtained with mutant recombinant enzymes rather than natural enzymes.