Boron-based Reductants
Mechanism + Description
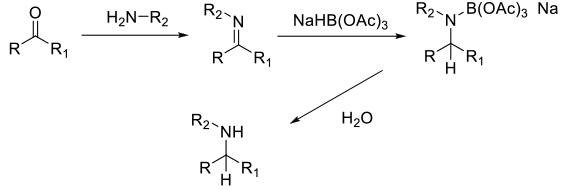
Delivery of a hydride from a BH reagent to reduce the imine or iminium intermediate. Generally not all hydrides transfer from the boron hydride source. Intermediate borates are hydrolysed to amines on work up.
General comments
NaBH4 is the cheapest and most readily available reagent, but due to instability in protic solvents, especially at lower pH, large excesses may be required. The reactivity of NaBH4 may also result in an unacceptable degree of background reduction of the ketone or aldehyde electrophile if the imine is not preformed. The most commonly used borates are NaH3BCN, and NaHB(OAc)3. The addition of electron–withdrawing groups making the reagents more stable to protic solvents and lower pH in which the imime may exist as the more reactive iminium ion. Borane:amine complexes, BH3:NR3 are occasionally employed, and silanes are sometimes used for the reduction of preformed imines.
Key references
J. Am. Chem. Soc., 1971, 93, 2897–2904 Cyanohydridoborate anion as a selective reducing agent
Relevant scale up examples
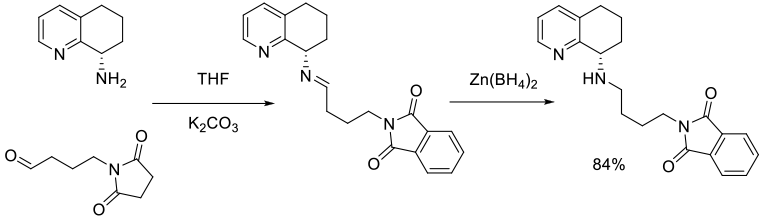
Org. Process Res. Dev., 2008, 12, 823–830
Experimental
50 g scale
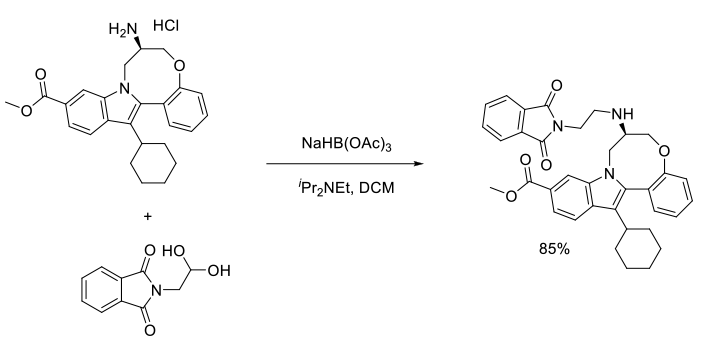
Org. Process Res. Dev., 2011, 15, 1116–1123
Experimental
7 kg scale
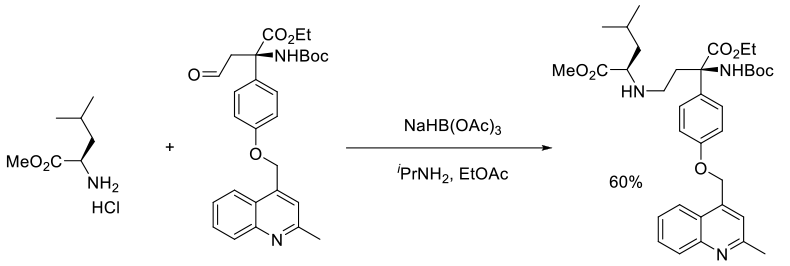
Org. Process Res. Dev., 2009, 13, 510–518
Experimental
112 kg scale
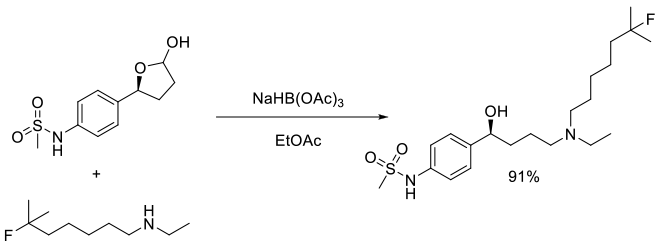
Org. Process Res. Dev., 2005, 9, 174-178
Experimental
5 g scale
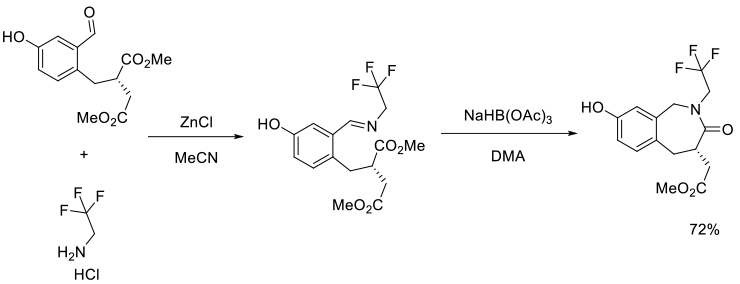
Org. Process Res. Dev., 2004, 8, 738-743
Experimental
56 kg scale
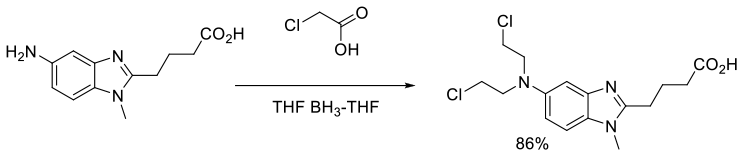
Org. Process Res. Dev., 2011, 15, 1063–1072
Experimental
400 g scale
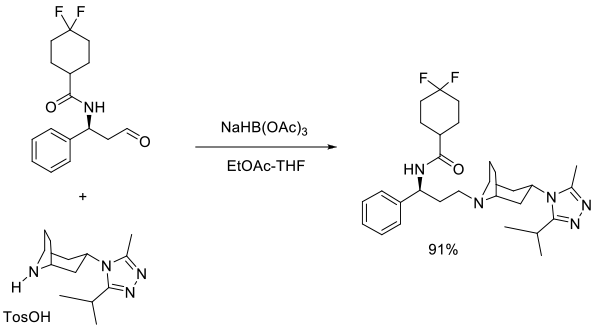
Org. Process Res. Dev., 2008, 12, 1104–1113
Experimental
65 kg scale
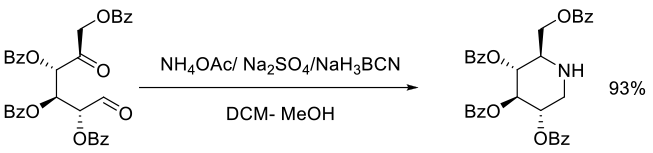
Org. Process Res. Dev., 2008, 12, 414–423
Experimental
13 kg scale
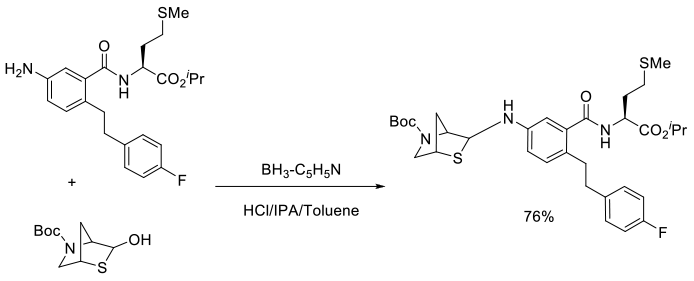
Org. Process Res. Dev., 2008, 12, 202–212
Experimental
73 kg scale
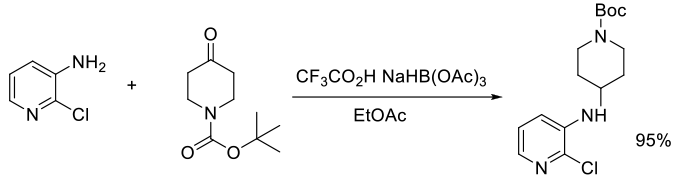
Org. Process Res. Dev., 2012, 16, 244−249
Experimental
500 g scale
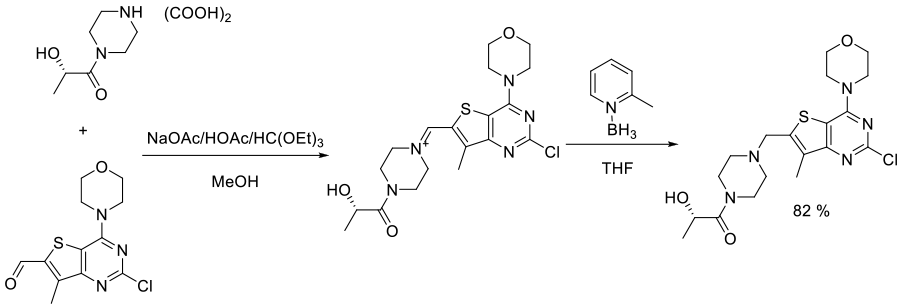
Org. Process Res. Dev., 2015, 19, 416−426
Experimental
9 kg scale
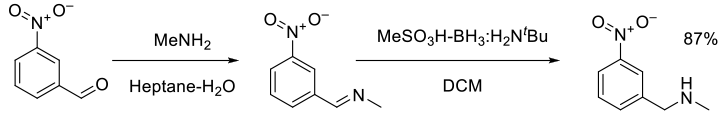
Org. Process Res. Dev., 2005, 9, 837-842
Experimental
70 g scale
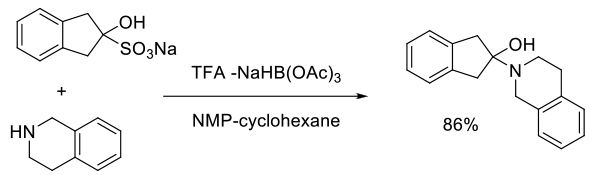
Org. Process Res. Dev., 2003, 7, 155-160
Experimental
1 g scale
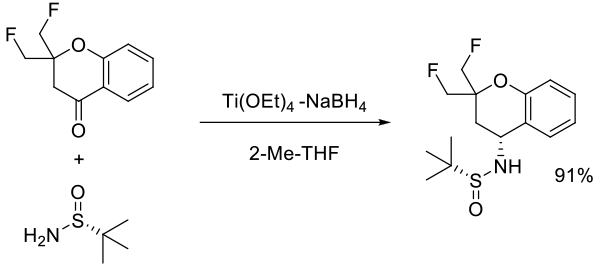
Org. Process Res. Dev., 2014, 18, 303−309
Experimental
30 kg scale
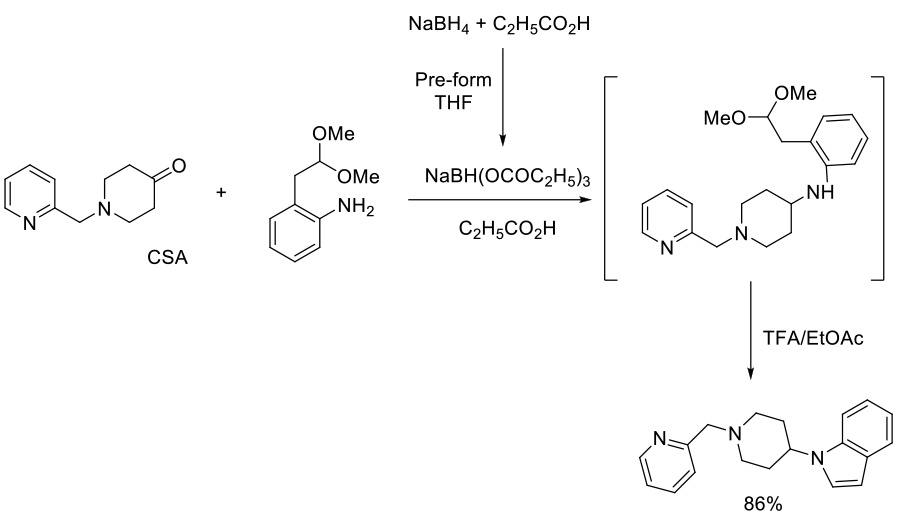
Org. Process Res. Dev., 2006, 10, 1205-1211
Experimental
200 g scale
Green Review
-
Atom efficiency (by-products Mwt)
Most boron-based reagents represent an atom inefficient way of delivering hydride with the other protic hydrogen coming from acid/solvent. In almost all cases, not all the hydrides on boron will be available for reaction. STAB is particularly atom inefficient, but widely used due to safer handling and enhanced selectivity. - Safety Concerns
Main issues are around toxicity, flammability, safe handling and storage. Care needs to be exercised with borane complexes (like BH3.THF) in that concentrated solutions are avoided. In some cases above ~ 1M, there can be considerable dissociation of BH3 forming an atmosphere of B2H6 in the reaction headspace. Flammable Hydrogen may be generated during reaction and work-up. Anionic borane complexes are more stable. NaH3BCN can generate very toxic gas on hydrolysis with acids
Org. Process Res. Dev., 2006, 10, 1292–1295 Safe Handling of Boranes at Scale
- Toxicity and environmental/aquatic impact
Boranes are generally highly toxic to mammals. Hydrolysis of boron-based reagents will lead to boric acid, which is a suspected reprotoxic mutagen. There may be issues with discharging aqueous waste with high boron content. Emerging data suggest boron compounds may be more ecotoxic that previously thought.
- Cost, availability & sustainable feedstocks
Borane reagents can be purchased in bulk (can be expensive per mole hydride) or made in situ at reasonable cost. There is no renewable source of borane reagents. - Sustainable implications
No great issues with Boron. With anionic complexes, Na and K are the preferred cations as Li is at medium risk of depletion. Some reactions are now being reported in biorenewable solvents like 2-methyl THF (see section on “specific solvent issues”).