Hydrogen/Metal Catalysts (Precious and Base Metal)
Mechanism + Description
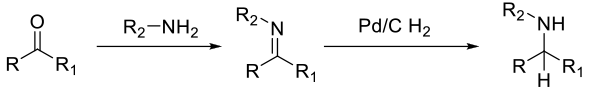
Typical heterogeneous reaction mechanism with the imine
intermediate ( formed in situ or preformed) binding to the
metal surface, then being reduced by hydrogen gas.
Mechanism shown for Pd/C
General comments
Typically the aldehyde and ketone are reacted under fairly traditional hydrogenation conditions – supported metal catalyst and H2 gas from atmospheric to ~ 100 psi. The most common metals are Pd and Pt, but other precious metals are occasionally used as well as cheaper base metals like Raney Nickel, Cobalt, Copper and Iron. Generally regarded as the greenest reductive amination since no borate by-products are formed, and down stream workup is simplified. Metal catalysts should be recovered and recycled and reaction products and waste effluent should be checked for metal content. Occasionally transfer hydrogenation is employed instead of H2 gas.
Homogeneous metals can be used as catalysts, but these are usually confined to chiral hydrogenation in the presence of chiral ligands to give enantioenriched amine products.
Key references
J. Am. Chem. Soc., 1948, 70, 2811-2812 Aminative Reduction of Ketones
J. Med. Chem., 1973, 16(5), 480-483. Asymmetric synthesis of psychotomimetic phenylisopropylaminesreductive amination with Pd/C catalyst
Tetrahedron, 2002, 58, 5669-5674 A modified palladium catalysed reductive amination procedure
See also catalysts for reductive amination in:
jmcct.com/crg/web/#/crg
Relevant scale up examples
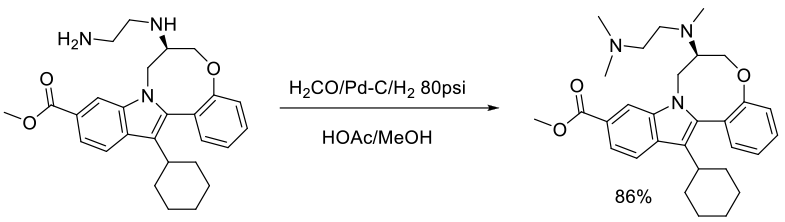
Org. Process Res. Dev., 2011, 15, 1116–1123
Experimental
5 kg scale
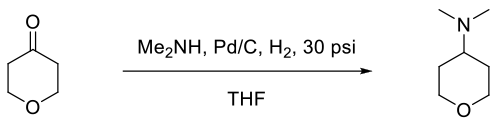
Org. Process Res. Dev., 2000, 4, 520-525
Experimental
3 kg scale
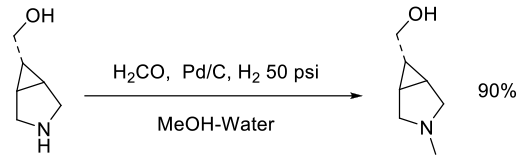
Org. Process Res. Dev., 2011, 15, 1052–1062
Experimental
3 kg scale
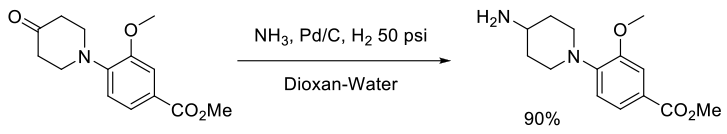
Org. Process Res. Dev., 2008, 12, 596–602
Experimental
300 g scale
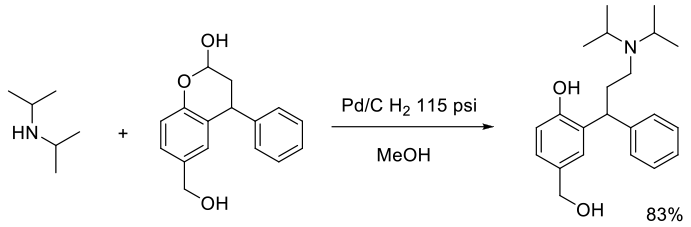
Org. Process Res. Dev., 2011, 15, 1010–1017
Experimental
400 g scale
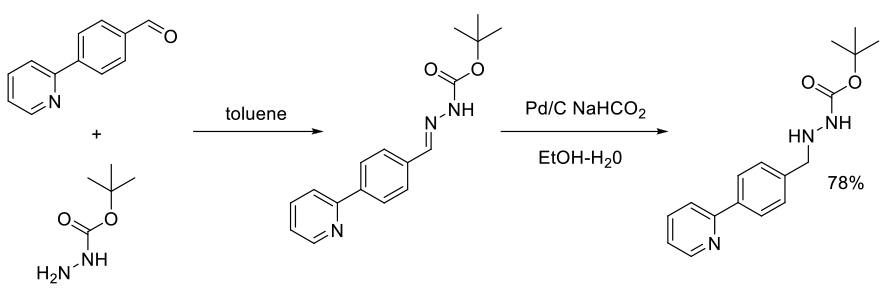
Org. Process Res. Dev., 2002, 6, 323-328
Experimental
112 g scale
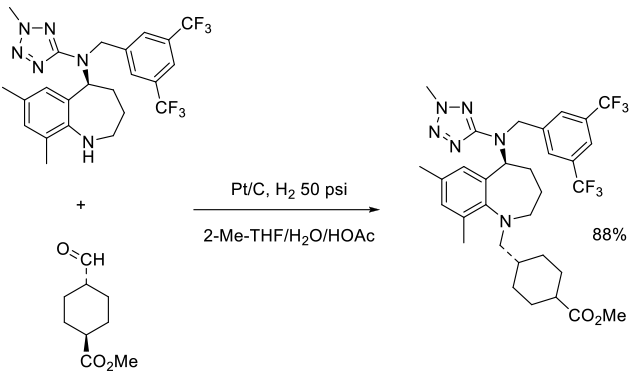
Org. Process Res. Dev., 2014, 18, 546–551
Experimental
250 kg scale
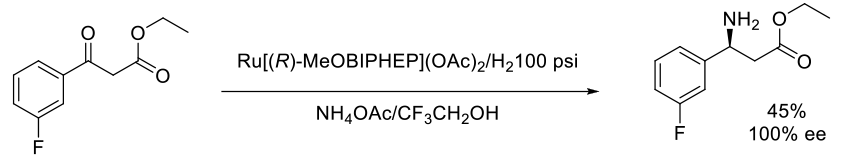
Org. Process Res. Dev., 2010, 14, 592–599
Experimental
4 kg scale
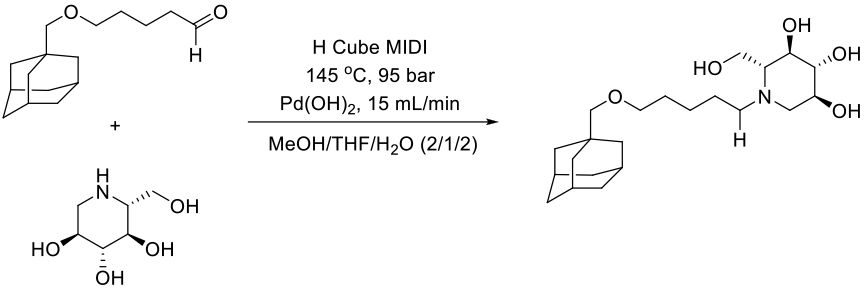
Org. Process Res. Dev., 2012, 16, 1090 – 1097
Green Review
-
Atom efficiency (by-products Mwt)
With optimized catalytic efficiency, hydrogenolysis is an atom efficient process using heterogeneous catalysts that can be recovered and recycled, or with homogeneous catalysts optimized for turnover number. - Safety Concerns
Typified by normal hazards around hydrogenation reactions. Catalytic transfer hydrogenation may avoid the need to handle H2 gas, but these reactions may generate H2 in situ. Heterogeneous metal catalysts can be pyrophoric in air when dry. - Toxicity and environmental/aquatic impact
Main concern is around solubilisation and loss of precious metal/heavy metal catalysts into waste streams. Most precious and heavy metal levels are tightly regulated. The same applies to potential carry through into the API. Some Ni salts are sensitizers and carcinogens and listed on the EU SVAH list – this is of less concern for metallic hydrogenation catalysts. - Cost, availability & sustainable feedstocks
H2 and H2 obtained via CTH (catalytic transfer hydrogenation) is cheap and non-polluting – can be from renewable resources. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with efficient recovery and recycle. Pd is most commonly used precious metal for hydrogenation, and this is rated at high risk of depletion. No concern for abundant base metals such as Ni.