Organo-catalysed Reductive Amination/alkylation
Mechanism + Description
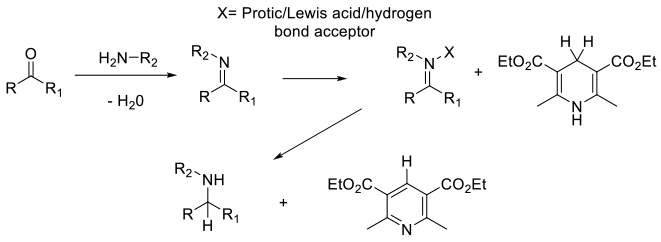
Formation of the imine which is activated by coordination / protonation followed by hydride transfer from an appropriate hydride donor.
General comments
Although not widely adopted for use at scale, an emerging area for reductive amination/alkylation is the use of organo-catalysed transformations. The reaction follows the normal reductive alkylation mechanism. The imine is formed and activated by a catalyst – usually Lewis/ Bronstead acidic, then reduced by hydride transfer from a donor. In the case of chiral organo-catalysts like phosphonic acids, chiral amine products can be accessed. The organo-catalysts active the imine intermediate by formation of iminium species by protonation, coordination, or hydrogen bonding. Typical organo-catalysts are: Thiourea, BINAP based phosphonic acids, isothiouronium chloride Typical hydride sources are : Hantzsch esters, benzothiazolines Key advantages are no metals are used and good compatibility with other functional groups: disadvantages are the poor atom economy associated with the commonly used hydrogen donors.
Key references
Synlett 2006, No. 6, 841–8440 Thiourea-Catalyzed Direct Reductive Amination of Aldehydes
J. Am. Chem. Soc. 2006, 128, 84 -86 Enantioselective Organocatalytic Reductive Amination
Relevant scale up examples
None found