Biocatalysis
Mechanism + Description
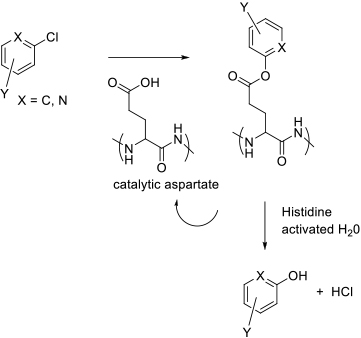 The mechanisms of enzymes that convert aryl and heteroaryl
halides to phenols are not all fully categorized, but some do seem to
catalyse true SNAr type reactions. These appear to have mechanisms
similar to haloalkane dehalogenases (catalytic triad Asp-His-(Asp/Glu)),
or are metalloproteases that use water activated by coordination to a metal
centre as the nucleophile.
The mechanisms of enzymes that convert aryl and heteroaryl
halides to phenols are not all fully categorized, but some do seem to
catalyse true SNAr type reactions. These appear to have mechanisms
similar to haloalkane dehalogenases (catalytic triad Asp-His-(Asp/Glu)),
or are metalloproteases that use water activated by coordination to a metal
centre as the nucleophile.
General comments
Whilst not an obvious reaction for bio-catalysis, a number of enzymes have been reported to give SNAr like conversions with aromatic and heteroaromatic halides. To date, only water has been used as the nucleophile, but rapid developments in evolving enzymes for promiscuous catalysis could facilitate use of other solvents.

Key references
Relevant scale up examples
None found.