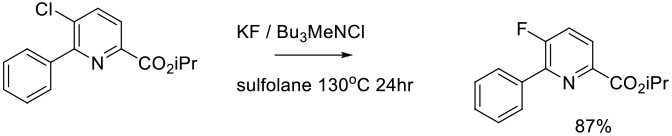
Halex Reaction
Mechanism + Description
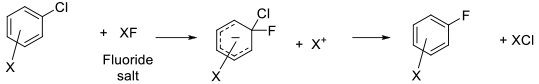
The Halex reaction is a specific SNAr reaction involving nucleophilic displacement of a leaving group (usually chloride) by a fluoride anion to generate the corresponding aryl/heteroaryl fluoride. The Halex reaction is widely used on scale to make fluorinated fine chemicals.
General comments
Typically Halex reactions are carried out at high temperature using dipolar aprotic solvents normally used for SNAr reactions. KF is the usual source of fluoride, although other more soluble fluoride sources like CsF or R4NF are sometimes used. Reactions are typically heterogeneous, and phase transfer catalysts are often employed to transfer fluoride anions into solution. As for SNAr reactions with N/O/S nucleophiles, the halex reaction works best with heteroaromatics and electron –deficient arenes.
Key references
Synthesis 2010 June 1; 2010(11): 1804–1821 C–F Bond Formation for the Synthesis of Aryl Fluorides
Relevant scale up examples solvents under pressure