Mechanism + Description
As per N-based dipolar aprotic solvents
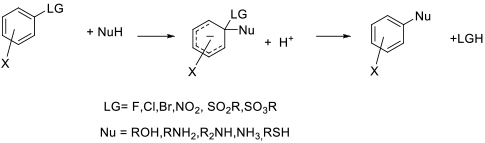
General comments
Some SNAr reactions will take place in water / aqueous mixtures provided that the nucleophile is significantly more reactive
than water which could compete to produce phenol by-products. Often, phase transfer catalysts or surfactants are added to aid
mixing and provide micelles where the organic reagents can dissolve and react. In the presence of chiral PTC, asymmetric
SNAr products can be obtained with certain reactants.
PTC’s can be added to less polar solvents to provide a more polar environment for reaction and to aid dissolution.
The use of PTC/surfactant systems in water can reduce the amount of organic solvent employed, but care needs to be taken with
disposal of any aqueous waste since some quaternary nitrogen / phosphorus based PTC’s can be quite ecotoxic.
Key references
European Journal of Organic Chemistry, 2017, 22, 3222-3228 Highly Regioselective Organocatalytic SNAr Amination of 2,4‐Dichloropyrimidine and Related Heteroaryl Chlorides
Org. Lett. 2017, 19, 194−197 Effects of Co-solvents on Reactions Run under Micellar Catalysis
Org. Lett. 2015, 17, 4734−4737 Nucleophilic Aromatic Substitution Reactions in Water Enabled by Micellar Catalysis
Org. Process Res. Dev., 2017 21, 218–221 SNAr Reactions in Aqueous Nanomicelles: From Milligrams to Grams with No Dipolar Aprotic Solvents Needed
RSC Adv., 2015, 5, 31226 SNAr reaction in aqueous medium in the presence of mixed organic and inorganic bases
Org. Process Res. Dev. 2009, 13, 230–241
J. Org. Chem. 1991 vol. 56;1041 – 1044 Micellar catalysis of organic reactions-SNAr reactions with neutral nucleophiles
ChemSusChem 2013, 6, 1455–1460 Amination of Heteroaryl Chlorides: Palladium Catalysis or SNAr in Green Solvents?
Heterocycles 1991, vol. 32; nb. 10,1947 – 1953 Easy and Efficient SNAr Reactions on Halopyridines in Solvent Free Conditions
Synthetic Communications; 1990 vol. 20; nb. 18; 2855 – 2864 Solid-Liquid Phase Transfer Catalysis Without Solvent: Further Improvement in SNAr Reactions
J. Org. Chem. 1998, 63, 9594-9596 A Simple and Efficient Method for the Preparation of Hindered Alkyl−Aryl Ethers
Eur. J. Org. Chem. 2002, 1278-1283 Solvent‐Free Microwave‐Assisted Aromatic Nucleophilic Substitution − Synthesis of Aromatic Ethers
J. Org. Chem., 2006, 71 Improved Asymmetric SNAr Reaction of β-Dicarbonyl Compounds Catalyzed by Quaternary Ammonium Salts Derived from Cinchona Alkaloids
J. Am. Chem. Soc., 2005, 127, 3670–3671 Organocatalytic Regio- and Asymmetric C-Selective SNAr Reactions
Relevant scale up examples solvents under pressure
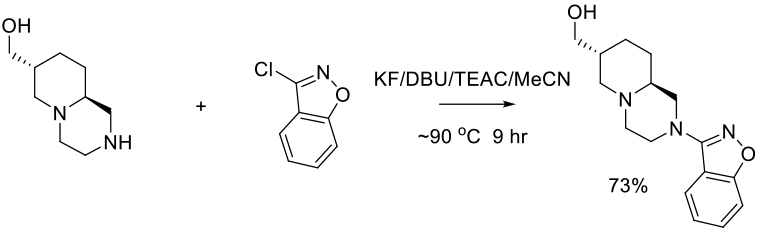
Org. Process Res. Dev. 2011, 15, 1328–1335
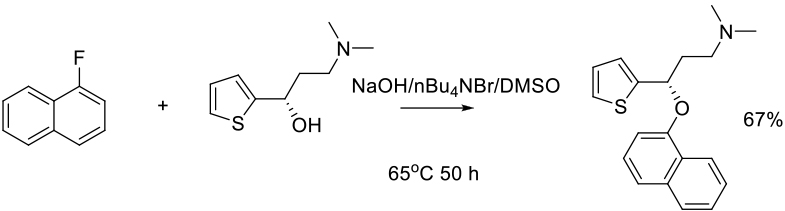
Org. Process Res. Dev. 2009, 13, , 854–856
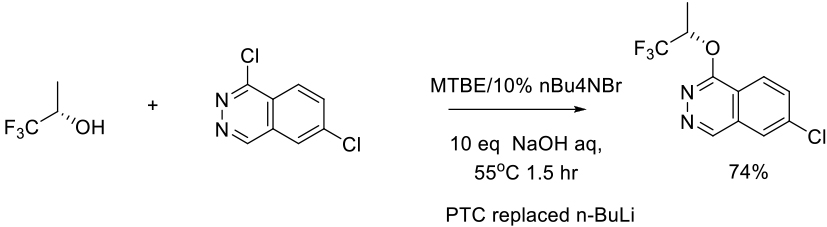
Org. Process Res. Dev. 2009, 13, 230–241
Org. Process Res. Dev. 2017, 21, 1286–1293




