Mechanism + Description
As per N-based dipolar aprotic solvents
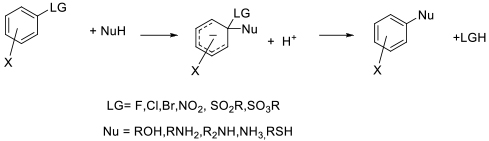
General comments
While many SNAr reactions will take place in solvents other than dipolar aprotics, the rates are often poor and too slow for practical application. One way to address this drawback is to use elevated temperatures with the solvent under pressure. This can often give reaction rates comparable with reaction in dipolar aprotic solvents. Heating in pressurized closed vessels is often associated with microwave heating, which can be used for smaller scale reactions. The use of continuous /flow chemistry with appropriate back pressure regulation can allow operation above a solvent’s boiling point and somewhat negates issues with running closed pressurized batch reactions at scale.
Operation at higher temperature can also be effective when a particularly unreactive electrophile or nucleophile is being employed.
Key references
Org. Bull. Chem. Soc. Japan 1991, 64, Issue 1 Pages 42-49 Aromatic Nucleophilic Substitution of Halobenzenes with Amines under High Pressure
Tet. Lett. 1999, 40, 2439-2442 The high-pressure SNAr reaction of N-p-Fluorobenzyl-2-chlorobenzimidazole with amines; an approach to norastemizole and analogues
Tet. Lett. 2016, 57, 2059–2062 CO bond formation in a microfluidic reactor: high yield SNAr substitution of heteroaryl chlorides
Tet. Lett. 2016, 57, 1035-1039 Nucleophilic aromatic substitution of heterocycles using a high-temperature and high-pressure flow reactor
Synlett; 2007, 14 . 2257 – 2261 Direct Uncatalyzed Amination of 2-Chloropyridine Using a Flow Reactor
Green Chem., 2011, 13, 794 A critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis
Appl. Organometal. Chem. 2012, 26, 273–276 Microwave‐promoted piperidination of halopyridines: a comparison between Ullmann, Buchwald–Hartwig and uncatalysed SNAr reactions
Chemistry Letters and Reviews 2012, Vol. 5, No. 4, 595-601 Efficient nucleophilic substitution of halopyridines using ethanol as solvent with microwave heating: synthesis of 2-aminoethylsulfanylpyridines
Relevant scale up examples solvents under pressure
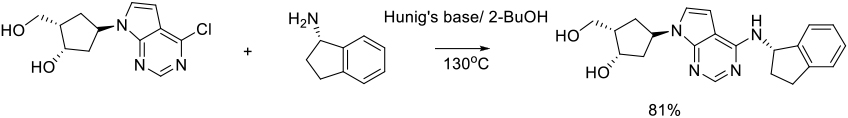
Org. Process Res. Dev. 2015, 19, 1299−1307
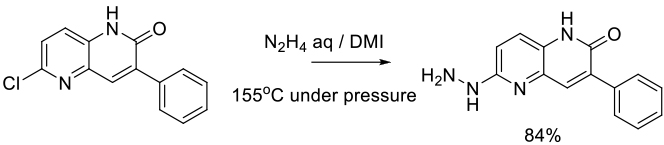
Org. Process Res. Dev. 2012, 16, 1069−1081
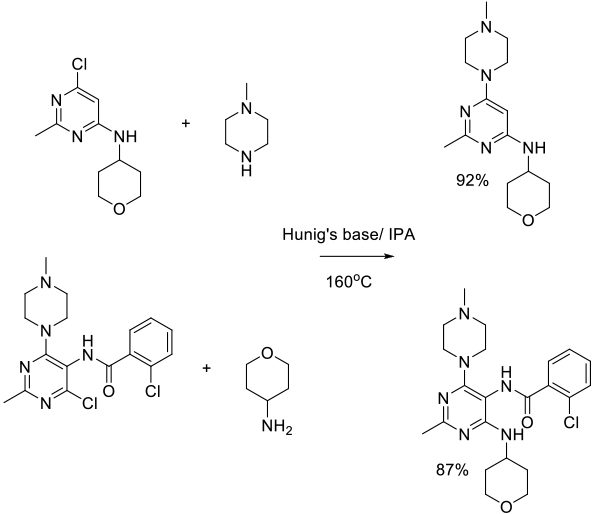
Org. Process Res. Dev. 2013, 17, 231−238
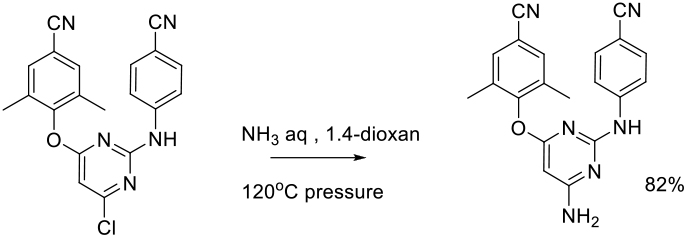
Org. Process Res. Dev., 2010, 14, 657-660




