SNAr Reaction in Other Common Molecular Solvents
Mechanism + Description
As per N-based dipolar aprotic solvents
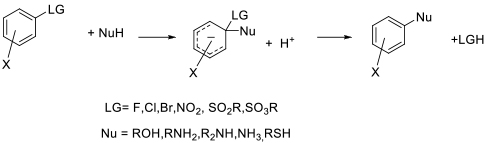
General comments
While dipolar aprotics are commonly used as solvents of first choice for SNAr reactions, many will take place in a range of solvents with better toxicity profiles. This is especially the case where more reactive electrophile/nucleophilepairs are used as reactants. Indeed, where the arene/heteroaromatic has two displaceable leaving groups, the use of a dipolar aprotic solvent often results in over reaction.
Esters
Some SNAr reactions can take place in esters like EtOAc , i-PrOAc, but if strong bases like NaOH, NaH, LDA, LHMDS, or alkoxides are needed, esters are not compatible.
Alcohols
Isopropyl alcohol, i– BuOH t-BuOH, isoamyl alcohol have been used, but some less hindered alcohols can act as nuclophiles in SNAr reactions.
Ethers
Glymes, THF, 2-Me-THF and dimethylisosorbide have all been used as solvents for SNAr reactions. Diglyme and related ethers have been identified as having reprotoxic properties and should be avoided if possible. 2-MeTHF has a much better LCA profile than THF, and is manufactured from biorenewable carbon sources. Dihydrolevoglucosenone (Cyrene) is promoted as a replacement dipolar aprotic solvent, but is unstable in the presence of bases, so may not be suitable for most SNAr chemistry.
Aromatics
Solvents like toluene are occasionally utilised as solvents in SNAr reactions. Occasionally small amounts of dipolar aprotic solvents are used as additives to increase reaction rate.
Liquid ammonia
Liquid ammonia has been proposed as an alternative to dipolar aprotic solvents for SNAr reactions
DOE/PCA
Design of experiments (DOE) and principal component analysis (PCA) have been used to select non toxic solvents to replace dipolar aprotic solvents
Key references
J. Am. Chem. Soc. 2000, 122, 712-713 Preparation of Tertiary Benzylic Nitriles from Aryl Fluorides
Relevant scale up examples in DMSO with Scheme
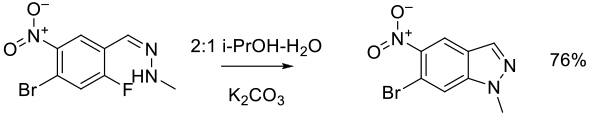
Org. Process Res. Dev. 2017, 21, 387–398
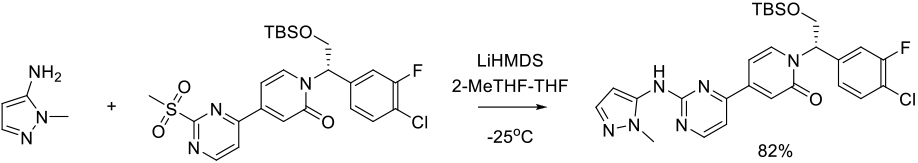
Org. Process Res. Dev., 2011, 15, 1073–1080
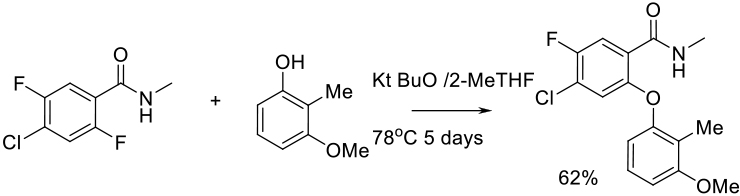
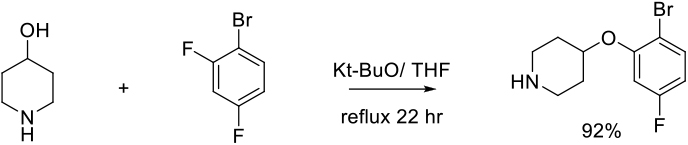
Org. Process Res. Dev. 2012, 16, 1805−1810
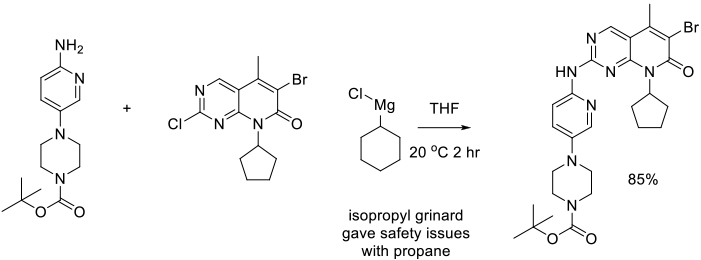
Org. Process Res. Dev., 2016 20, 1191–1202
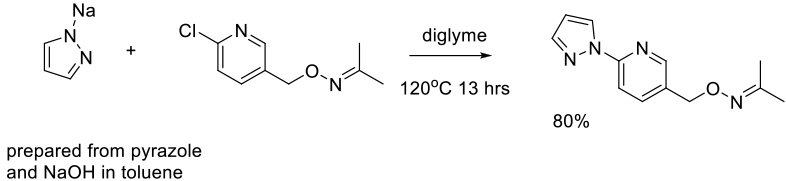
Org. Process Res. Dev. 2012, 16, 220−224
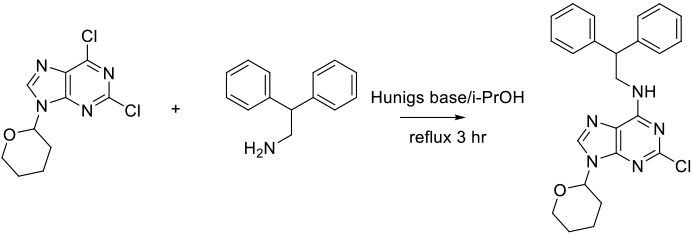
Org. Process Res. Dev. 2008, 12, 575–583
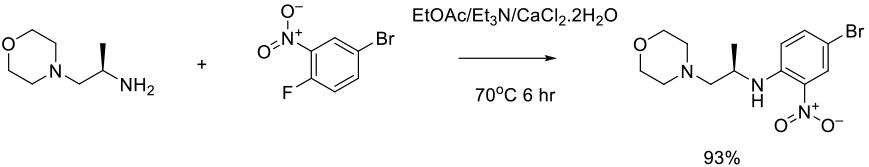
Org. Process Res. Dev. 2017, 21, 208−217
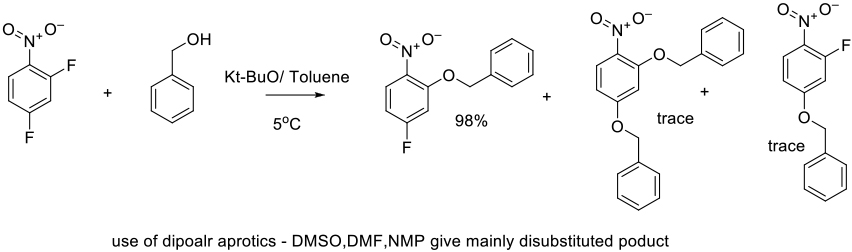
Org. Process Res. Dev., 2014, 18, 912–918
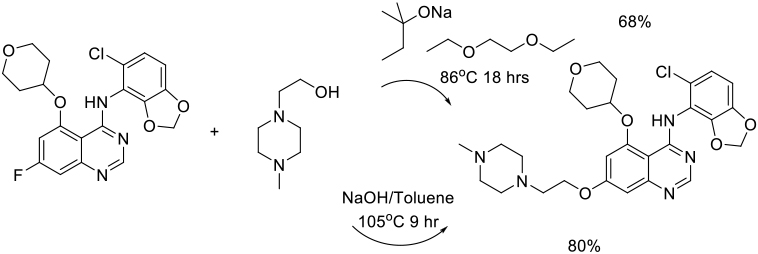
Org. Process Res. Dev. 2011, 15, 688–692
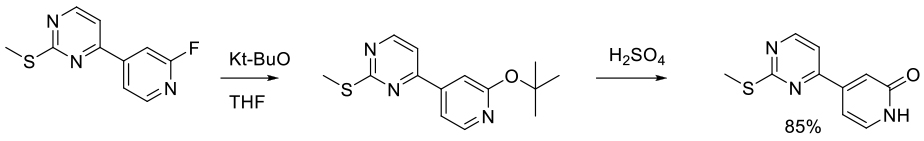
Org. Process Res. Dev. 2017, 21, 1320−1325
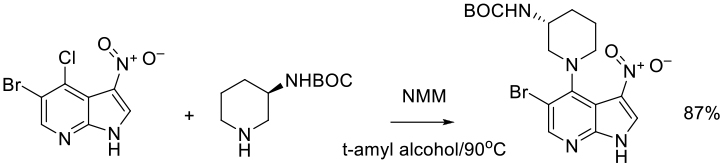
Org. Process Res. Dev., 2018, 22 (3), pp 344–350