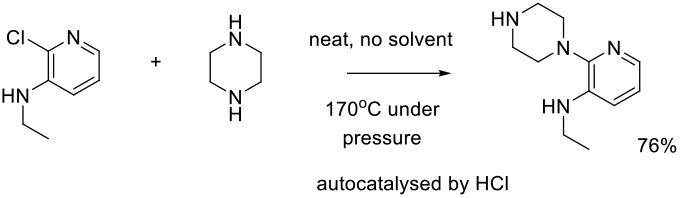
SNAr Reaction Run Neat
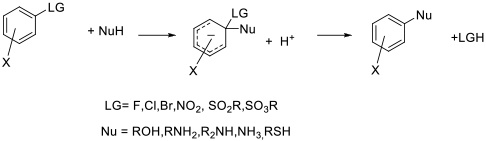
Mechanism + Description
As per N-based dipolar aprotic solvents
General comments
If one reagent is cheap and readily available, and can be used in excess, or if the reaction mixture forms a mobile liquid at reasonable temperature, there may be a possibility to run the reaction without solvent. This does somewhat detract from PMI metrics if the excess reagent is not recovered, and care needs to be taken regarding any potential exotherms and how they are handled with a diluent present. Neat reactions can be run at atmospheric or elevated pressure.
Phase transfer catalysts (PTC) are sometimes added to aid reaction –see section on PTC
Key references
Relevant scale up examples solvents under pressure