Jacobsen Asymmetric Epoxidation
Mechanism + Description
The mechanism is not fully understood. The possibilities are a concerted pathway, a metalla-oxetane pathway and a radical pathway.
General comments
The Jacobsen epoxidation and Katsuki variant are more flexible than the Sharpless AE since the presence of an allylic alcohol function is not required. A chiral Mn salen complex is oxidised and the oxygen transferred to one prochiral face of the alkene. A variety of oxidising agents can be employed as the terminal oxidant – mCPBA, NaOCl etc.
Key references
Short review of Jacobsen epoxidation
J. Am. Chem .Soc.112 (7): 2801–2803 Jacobsen paper
J . Org. Chem. 1994 59 (16): 4378–4380 Enantioselective, Catalytic Epoxidation of Trisubstituted Olefins
Tet. Asymm. 1991,2, 481-498 Catalytic asymmetric epoxidation of unfunctionalized olefins
Relevant scale up example
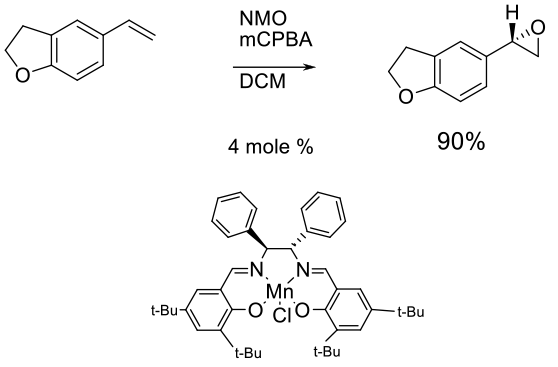
Experimental
850 gram scale
Org. Process Res. Dev. 2003, 7, 821-827
Green Review
-
Atom efficiency (by-products Mwt)
In order to achieve good atom efficiency, the quantities of the terminal oxidant should be optimised, a low Mwt oxidant like NaOCl employed. Generally better catalytic efficiency is seen with the Jacobsen compared to the Sharpless epoxidation. - Safety Concerns
Main issues are around the terminal oxidant – especially if a peracid like mCPBA – see peracids section. The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process Safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have destructive consequences, it is important to ensure that active oxidants are not present in waste streams. Use of an alternate solvent may avoid such safety concerns. - Toxicity and environmental/aquatic impact
Typically run in DCM, other greener solvents can be employed for the Jacobsen epoxidation. - Cost, availability & sustainable feedstocks
Reasonably cheap and readily available reagents. Biggest cost is the chiral Mn catalyst. - Sustainable implications
Manganese is rated at medium risk for depletion