From 1,2 diols
Mechanism + Description
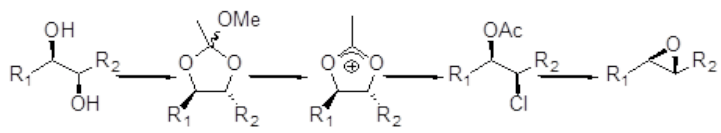
‘one pot’ conversion of diols to epoxides
General comments
A related reaction is epoxide formation from 1,2 diols. Chiral 1,2-diols, from the Sharpless asymmetric dihydroxylation (AD) reaction (or other sources), are converted to the cyclic orthoacetate, ring opened to give the acetoxonium intermediate followed by base mediated ring closure to furnish the epoxide. This method is complementary to the Sharpless asymmetric epoxidation (AE) protocol, but is applicable to a wide range of suitable substrates (all olefins), rather than the specific allylic alcohols required for the Sharpless AE. A one-pot procedure, albeit with several steps.
Key references
Tetrahedron, 1992, 48, 10515-10530 Sharpless paper
Chem. Rev. 1994, 94, 2483–2547 Catalytic Asymmetric Dihydroxylation a review
J. Am. Chem. Soc., 1988, 110 1968–1970 Asymmetric dihydroxylation via ligand-accelerated catalysis
Relevant scale up example
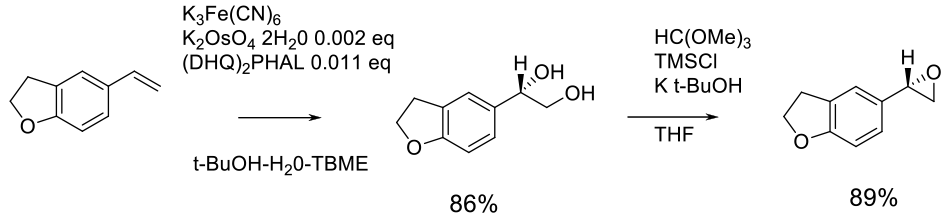
Experimental
15 kg scale
Org. Proc. Res. Dev., 2003, 7 (6), pp 821–827
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficacy is really dependent on the reagents used to form the epihalohydrin and ring closure reactions. Ring closure of epichlorohydrins and epibromohydrins is preferred over making sulfonates from diols. - Safety Concerns
No major risks apparent with halogenation and ring closure reactions. Sharpless methodology using Os needs special handling due to the toxicity of Osmium reagents and the potential to stain processing equipment with ‘Osmium black’. A number of supported and encapsulated Os catalysts can be accessed to minimize these issues. - Toxicity and environmental/aquatic impact
No major concerns with most reagents. TMSCl is often used as a reagent in the ring closure the by-product, (TMS)2O, is poorly biodegraded and can accumulate. - Cost, availability & sustainable feedstocks
Most reagents are cheap and readily available apart from Os. - Sustainable implications
Os-catalysed dihydroxylations – Os is rare and classed at high risk of depletion. Production has a high LCI.