Cyanide
Mechanism + Description
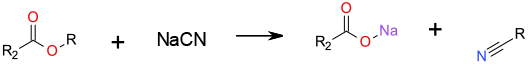
SN2 displacement with sodium cyanide to give an organic nitrile and salt of the acid.
General comments
Esters can be cleaved using sodium or potassium cyanide in dipolar aprotic solvents to yield the corresponding carboxylic acid and the nitrile analogue of the alcohol (SN2 attack liberating the carboxylic anion). The reaction is dependent upon the structure of ester used. Methyl and ethyl esters are generally preferred substrates. Whilst HMPA is the preferred solvent in terms of the rate of reaction, other dipolar aprotic solvents (DMF) have been used. Potassium cyanide has been used sd a mild trans-esterification catalyst suitable for acid or base-sensitive compounds. The use of cyanides is restricted under a number of EU and US regulations. Workup involving acids can result in the liberation of hydrogen cyanide gas. Early works reference the use of HMPA – The use of HMPA is severely restricted under a number of EU and US regulations. Other dipolar aprotic solvents like DMF may be listed on the EU REACH SVHC list due to reprotoxicity properties. Aqueous cyanide wastes are highly toxic to eco systems.
Key references
Tet. Lett., 1973, 3565 Decarboxylation of β-ketoesters in hexamethylphosphoric triamide
Helv. Chim. Acta, 1974, 57, 987 SN2 Reactions with Carboxylic Esters. Selective cleavage of methyl esters
Synthesis, 1973, 790A mild transesterification Method
Relevant scale up example
None found