Peptide Synthesis – Safety Topics
A number of issues have been highlighted with chemistry associated with peptide synthesis.
Solvents
The use of undesirable CMR solvents have been discussed in the main text.
Sensitization by Acid Activating Reagents
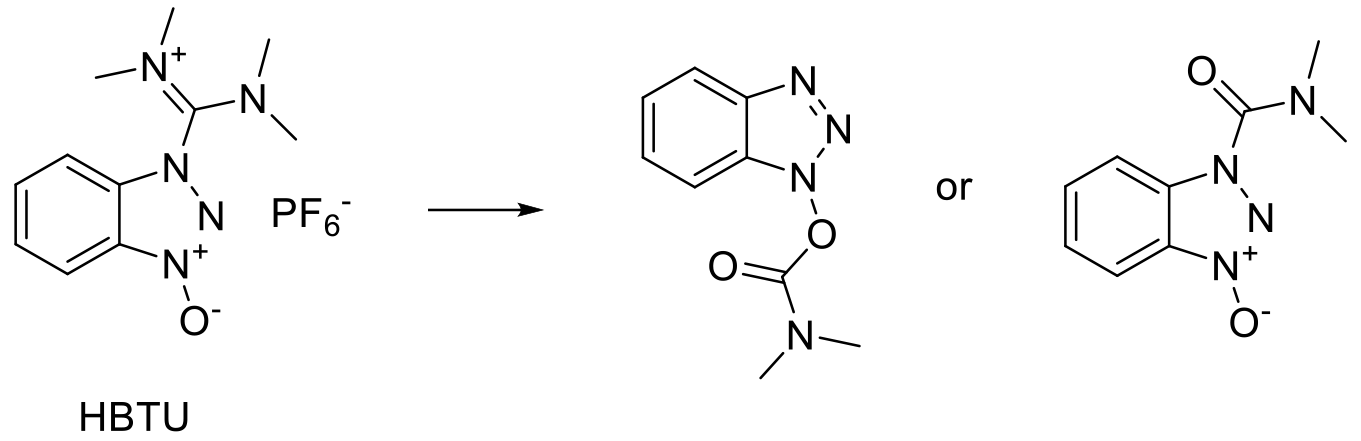
A number of classical acid activators used in peptide synthesis such as DCCI, HATU, HTBU and HCTU have been found to be sensitizers and should be labelled, stored and handled to avoid this hazard. Individuals with known chemical sensitization should be made aware these materials are being used and excluded from the facilities.
Thermal Stability of Peptide Coupling Reagents
A recent study has shown a total of seven reagents could be flagged as potentially shock sensitive. Of these seven reagents (TDBTU, TBTU, HDMA, HATU, TCTU, PyBOP, and NDSC), four (TDBTU, HDMA, HATU, and TCTU) exhibited discoloration during the drop hammer experiments. A total of 12 reagents were flagged as potentially explosive (TDBTU, HDMA, HATU, TCTU, PyBOP, NDSC, TOTU, HBTU, PyAOP, DMTMM, TNTU, and TPTU). Out of these 12 reagents, one tested as potentially Class 1 explosive by Scan ARC analysis. the authors put forward the following recommendations.
Most Preferred |
Use with Caution |
Least Preferred |
||
MsCl |
EDCI |
ECF |
HCTU |
|
TsCl |
DIC |
IBCF |
TCTU |
|
POCl3 |
DCC |
TSTU |
TPTU |
|
(COCl)2 |
PyBrOP |
DMTMM |
COMU |
|
T3P |
PyClOP |
BOPCl |
HATU |
|
CDI |
PyClU |
TNTU |
||
PivCl |
DppCl |
HDMA |
||
EEDQ |
DEPC |
NDSC |
||
IIDQ |
DPP |
TDBTU |
||
BBDI |
DPC |
TBTU |
||
TCT |
FDPP |
HBTU |
||
CDMT |
CIP |
TOTU |
||
CITU |
TFFH |
|||
Thermal Hazards with 1-Hydroxybenztriazole
This reagent is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal). Anhydrous HOBt is explosive. Once commonly used in peptide synthesis, issues with safety and shipping have led to other reagents being developed.
Cyanide Generation from OPTIMA
OPTIMA was devised as a replacement for HOBt and has been widely employed. Recent studies have highlighted the safety hazard of cyanide generation when used with reagents like carbodiimides like DIC, DCCI, etc. (see scheme below).
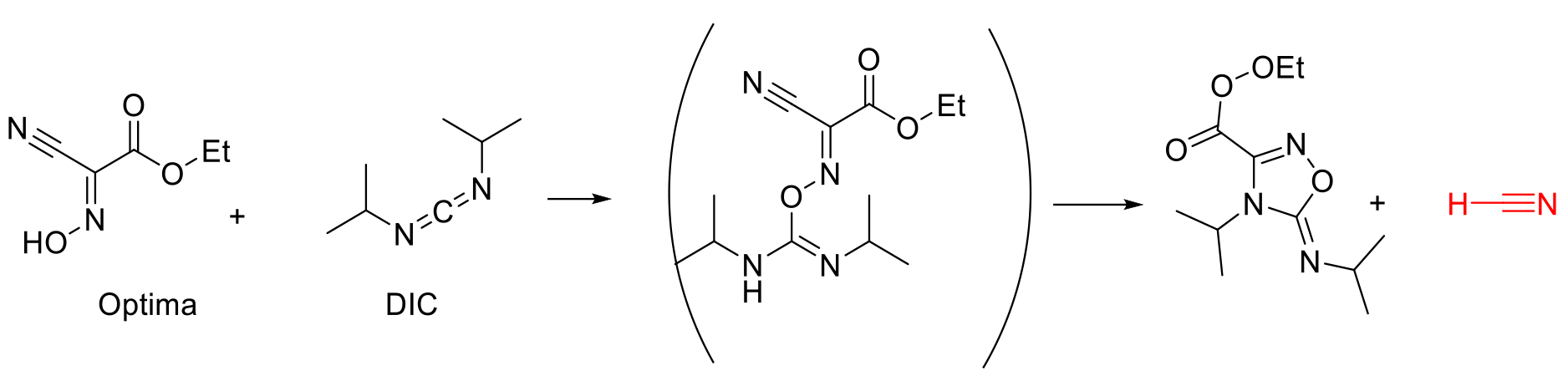
The rate of formation of HCN is dependent on the solvent composition and the reagents ratios, and among the greener alternatives to DMF, NPB/EtOAc (1:4) was found to minimize the formation while accelerating the coupling kinetics when employed with a 1:1:1 ratio of amino acid/Oxyma/DIC. HCN scavengers like DimethylTrisulfide (MeSSSMe).
A recent publication highlighted the formation of a novel impurity formed by the decomposition of HBTU in the presence of NMM when using MeCN, DMF or NMP as solvents. The compound could be classed as a PGI, so caution is advised if used towards the end of a c-GMP synthesis.