Downstream Processing/ Purification/ Isolation of Peptides
Many peptides are traditionally purified and isolated by chromatography and freeze drying/lyophilization. Depending on purity required, multiple chromatography operations may be needed. Large-scale chromatography remains a significant cost center, and buffers and solvents used for eluents can give a large PMI for the product. Lyophilization is energy intensive on a large scale.
Depending on purity requirements and stability characteristics, more mass efficient and less energy intensive technologies like multi-column chromatography and spray drying might be considered.
Affinity tags/peptide ‘easy clean’ technology
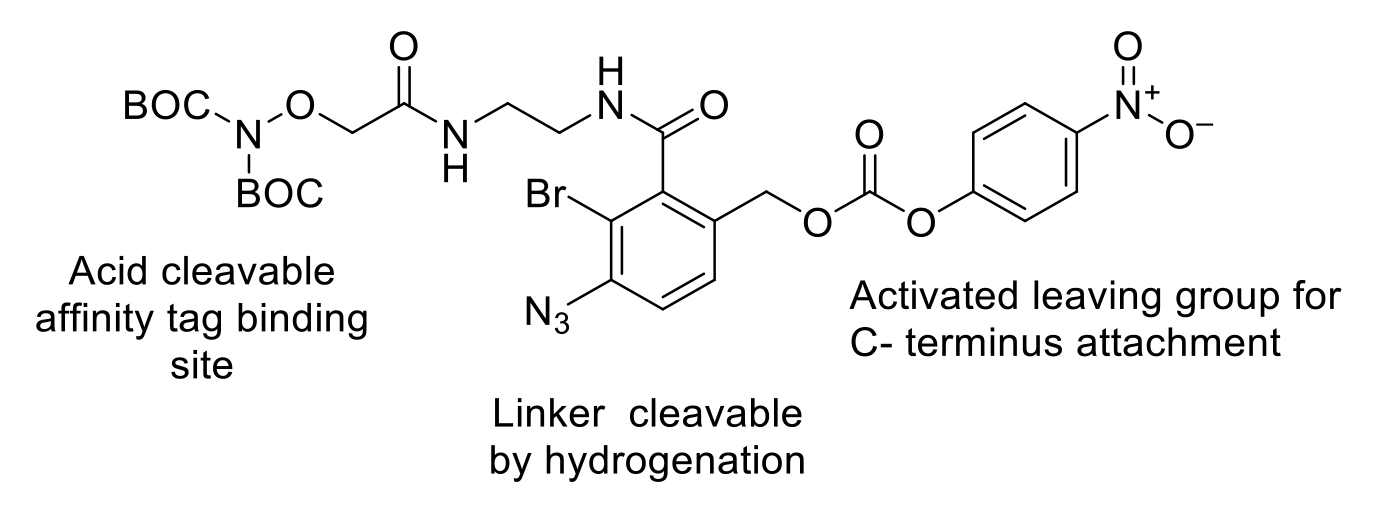
Traceless affinity tagging is another emerging technique to provide alternative, more selective, and sustainable isolation of peptides. There are several strategies that can be employed. Typically, a multi-functional tag is attached to the peptide. Also present are other groups that can be manipulated with orthogonal deprotection. In the case of the example below, acid cleavage exposes a hydroxylamine that can be used as a binding site for an affinity chromatography resin. Once purified, the affinity group and resin attachment group are removed by hydrogenation.
Relevant Scale Up Examples
Kilogram-Scale GMP Manufacture of Tirzepatide Using a Hybrid SPPS/LPPS Approach with Continuous Manufacturing
A hybrid solid-phase peptide synthesis/liquid-phase peptide synthesis (SPPS/LPPS) approach for the synthesis of tirzepatide. Continuous manufacturing and real-time analytical monitoring give high-quality material. The use of nanofiltration avoided difficult precipitations during intermediate purification.
Org. Process Res. Dev. 2021, 25, 1628–1636.
9 kg scale
Copper(II) Lysinate and Pseudoproline Assistance in the Convergent Synthesis of the GLP‑1 Receptor Agonists Liraglutide and Semaglutide.
The synthesis of lipopeptides liraglutide and semaglutide using a stepwise condensation of three fragments in SPPS. The use of pseudoproline and copper(II) lysinate intermediates was also described.
Org. Process Res. Dev. 2021, 25, 1598–1611.
1g scale
Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS).
H-22-39-NH2 and H-1-21-O-Cam-L-NH2 exenatide fragments for the enzymatic ligations were synthesized by SPPS methods and ligated by omniligase-1 enzyme.
Green Chem. 2019, 21, 6451-6467.
25 g scale
Improved Tag-Assisted LPPS: An Application to the Synthesis of Bradykinin Receptor-Antagonist Icatibant Acetate.
Soluble tag-assisted liquid-phase peptide synthesis using a hydrophobic benzyl alcohol as a support is applicable to the 100 g scale.
Org. Process Res. Dev.. 2019, 23, 2576–2581.
100g scale
Org. Process Res. Dev.. 2012, 16, 1279−1282.
1.4 kg scale flow process