Carbonyldiimidazole (CDI)
Mechanism + Description
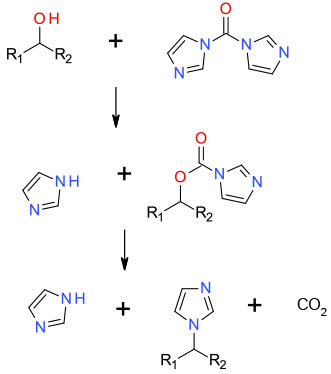
Activation of the alcohol as a mixed carbamate followed by displacement with liberated imidazole
General comments
For a limited range of alcohols and its thio-analogue, has been shown to convert alcohols directly to N-alkylated imidazoles. If certain structural requirements are met, chiral integrity is maintained. Triazole versions of CDI also undergo this transformation. For amino alcohols where cyclization is possible, CDI has been reported as a greener alternative to Mitsunobu or Appel reagents for ring formation.
Key references
J. Org. Chem. 1997, 62, 7319-7323 Imidazole Transfer from 1,1-Carbonyldimidazole and 1,1¢-(Thiocarbonyl)diimidazole to Alcohols. A New Protocol for the Conversion of Alcohols to Alkylheterocycles
J. Org. Chem. 2004, 69, 5124-5127 Regio- and Stereospecific Syntheses of from the Reaction of 1,1¢-Carbonyldiimidazole with syn– and anti-1,2-Amino Alcohols
J. Org. Chem. 2006, 71, 4147-4154 N,N-Carbonyldiimidazole-Mediated Cyclization of Amino Alcohols to Substituted Azetidines and Other N-Heterocycles
Relevant scale up example
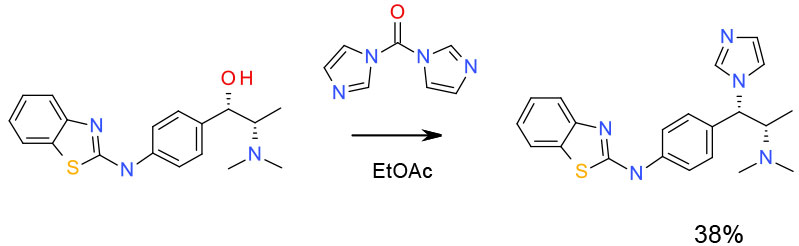
Experimental
100g scale- heat in EtOAc
Org. Process Res. Dev. 2001, 5, 467-471
Green Review
-
Atom efficiency (by-products Mwt)
Reaction generates one mole of CO (28) and 1 mole imidazole (68) as by-products, so medium atom efficiency. - Safety Concerns
No major concerns. Reactions will generate carbon dioxide off gas. - Toxicity and environmental/aquatic impact
Reagent decomposes to innocuous products. Imidazole is emerging as a material with ECHA review to categorize due to emerging reproductive toxicity issues. - Cost, availability & sustainable feedstocks
CDI is readily available and a cheap reagent – most often used for amide formation. - Sustainable implications
More sustainable than the Mitsunobu reaction, but currently limited in scope. Possibly a good choice for ring formation.