Direct Alcohol Activation
Mechanism + Description
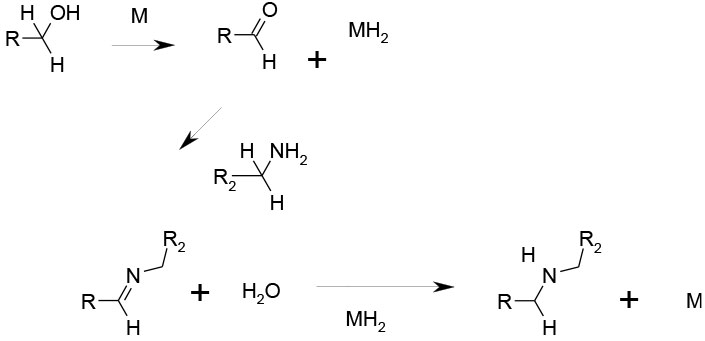
An alcohol is oxidised in situ to the aldehyde by a metal Catalyst – usually Ir or Ru. This forms an imine with an amine which is then reduced by the reduced catalyst to the alkyl amine and the oxidised form of the catalyst – this is often referred to as ‘borrowing hydrogen’.
General comments
Currently an area of great interest – direct coupling of alcohols with amines via intermediate aldehyde and imine generated in situ via ‘borrowing hydrogen’. The usual metal catalysts are based on Ir or Ru complexes. The use of appropriate diols and amines can lead directly to heterocyclic rings. Detractions are that chiral centres at the alcohol are lost in the transformation, and high levels of metal catalysts like Ir add high cost to the process and issues with removing metal downstream. As with most other branches of metal-catalysed reactions, there is currently a great deal of interest in replacing precious metals with base metals, or indeed non-metallic catalysts. Some relevant recent references are given below.
Gold catalyzed Alcohol Activation for Substitution Reactions
An emerging method to catalytically activate alcohols for substitution reactions- Very limited application to C-N bond formation of propargylic alcohols. Apparently proceeds via a cationic mechanism; stereochemistry of chiral alcohols is lost.
Key references
Chem. Eur. J. 2010, 16, 13193 – 13198 New Iridium Catalysts for the Efficient Alkylation of Anilines by Alcohols under Mild Conditions
Angew. Chem. Int. Ed. 2010, 49, 8126 –8129 An Efficient and General Synthesis of Primary Amines by Ruthenium- Catalyzed Amination of Secondary Alcohols with Ammonia
Angew. Chem. Int. Ed. 2010, 49, 8130 –8133 Direct Amination of Secondary Alcohols Using Ammonia
ChemCatChem. 2011, 3(9), 1407-1409 Highly Efficient and Selective Catalytic N-Alkylation of Amines with Alcohols in Water
J. Am. Chem. Soc. 2009, 131, 1766-1774 Ruthenium-Catalyzed N-Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology
Borrowing hydrogen –base metal catalysis
Angew. Chem. Int. Ed. 2008, 47, 8661-8664. Selective Synthesis of Primary Amines Directly from Alcohols and Ammonia
Eur. J. Org. Chem. 2009, 5383–5389 Amination of Alcohols Catalyzed by Copper-Aluminium Hydrotalcite: A Green Synthesis of Amines
Angew. Chem. Int. Ed. 2011, 50, 3006 –3009 Iron/Amino Acid Catalyzed Direct N-Alkylation of Amines with Alcohols
ChemCatChem 2011, 3(12), 1853-1864 review -The Catalytic Amination of Alcohols
Dalton Trans. 2009, 753-762 Transition metal catalysed reactions of alcohols using borrowing hydrogen methodology
J. Org. Chem. 2009, 74, 2877-2879 One-Pot Synthesis of Imines and Secondary Amines by Pd-Catalyzed Coupling of Benzyl Alcohols and Primary Amines
Adv. Synth. Catal. 2013, 355, 73 – 80 Green and Scalable Aldehyde-Catalyzed Transition Metal-Free Dehydrative N-Alkylation of Amides and Amines with Alcohols
Borrowing hydrogen –Gold catalysis
Curr. Org. Synth. 2008, 5, 28-32. Gold-catalyzed propargylic substitutions: Scope and synthetic developments
Relevant scale up example
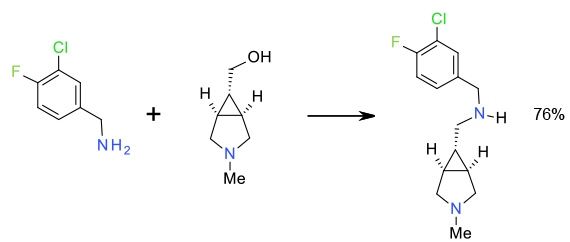
Experimental
Toluene/H20-NaHCO3/
(Cp*IrCl2)2 2.5 Kg scale
Org. Process Res. Dev. 2010, 14, 441–458
Green Review
-
Atom efficiency (by-products Mwt)
An atom efficient transformation to make amines generating H2O (18) as a by-product. - Safety Concerns
No major operational hazards are apparent. For some catalytic systems, care should be taken in case hydrogen is generated in the headspace above the reaction. Reactions requiring the presence of O2 / air can generate a flammable atmosphere. - Toxicity and environmental/aquatic impact
Generally low impact with low catalytic loadings. As with all heavy metals, contamination of the environment needs to be avoided and metal levels in the final API need to be controlled. Non-toxic base metals are preferred. - Cost, availability & sustainable feedstocks
Cost here equates to metal used and catalytic efficiency. Precious metals should be recovered and reused. - Sustainable implications
Most precious metals are at risk of depletion –although at an early stage of adoption, base metal catalysts are preferred over Pd/Ir/Ru ( high risk of depletion)