Base Mediated Displacement
Mechanism + Description
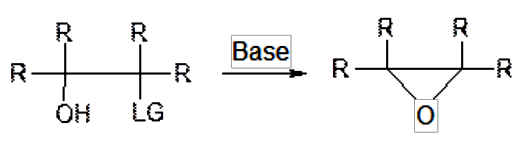
Intramolecular ring closure of alcohol α to a suitable leaving group.
General comments
Generally scalable epoxide formation by SN2 displacement of a leaving group adjacent to a hydroxyl function. Widely applicable for a range of substrates. May not be suitable where other base sensitive groups are present. For the closure of halohydrins, the reaction is often combined with the oxidation of the alkene with halogen reagents and acids/PTC’s. Epoxidations with bleach (NaOCl) normally occur via formation of the chlorohydrin and in situ closure to the epoxide.
Key references
Adv. Synth. Catal. 2003, 345, No. 3, 389 Convenient Method for Epoxidation of Olefins without Metal Catalysts
Org. Synth. Coll. Vol. 8, 1993, 434-442 (1988, 66, 160-168) example of base epoxidation.
J. Am. Chem. Soc., 2004, 126, 6844-6845 chiral catalysts for the highly enantioselective epoxidation of α,β-unsaturated ketones
Relevant scale up example
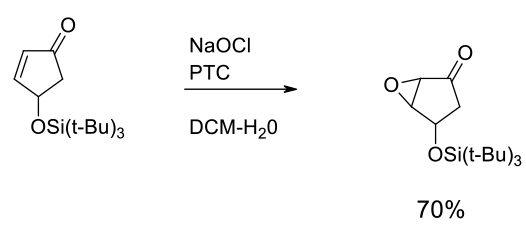
Experimental
Gram scale
Org. Process Res. Dev. 2009, 13, 1111–1121
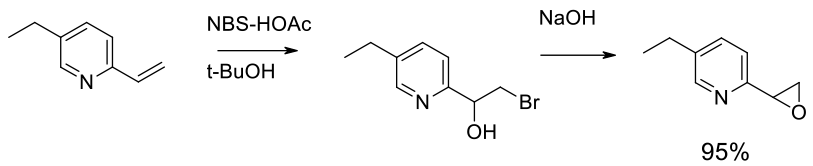
Experimental
82 kg scale
Org. Process Res. Dev. 2002, 6, 721- 728s
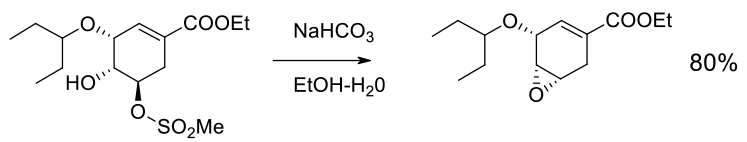
Experimental
3 kg scale
Org. Process Res. Dev. 2002, 6, 721- 728
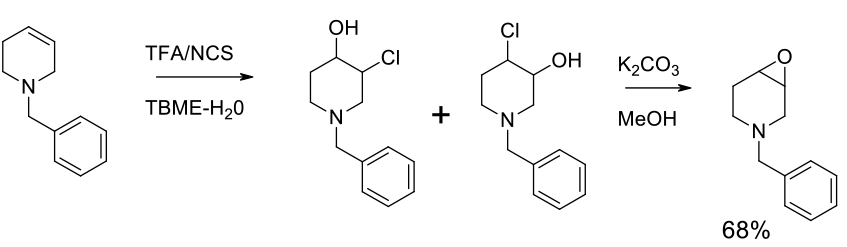
Experimental
1 kg scale
Org. Process Res. Dev. 2012, 16 1558–1565