Biocatalysis
Mechanism + Description
Direct epoxidation
The active Fe/Mn residue is oxidised by O2 to a high valent metal oxo species which then transfers O to the alkene. Since the reaction occurs in the enzyme active site, the products from prochiral alkenes often show high ee.
In situ generation of peracids
Esters or acids form an acyl intermediate with serine in the active site of a lipase or protease. This intermediate reacts with H2O2 to generate a peracid, which can react with the alkene in the normal way, and recycle the acid catalyst. Care needs to be taken with the levels of peroxide – high concentrations can denature the enzyme.
General comments
A number of biocatalytic methods exist for the synthesis of epoxides. Olefins can be directly epoxidised by monooxygenase p450 and peroxidase enzymes. In this case, the terminal oxidant is O2. Generally isolated p450 enzymes are just too expensive and unproductive for preparative use other than small scale metabolite confirmation/synthesis. Whole cell reactions can be more productive, but are still often very volume inefficient. Peroxidases probably have more promise for scale up, but care needs to be taken with peroxide concentrations and enzyme deactivation.
Certain hydrolase enzymes – lipases and proteases – can be used as catalysts to generate peracids in situ from H2O2 and acids or esters. Strictly this is not an enzyme – catalysed oxidation since the actual epoxidation occurs remotely from the active site.
Key references
Whole cell epoxidations
ChemBioChem, 2008 9(7), 1116-1123; Stereoselective alkene epoxidation
Chirality, 1989, 1(2), 127-36; Cytochrome P-450-catalyzed asymmetric epoxidation of simple prochiral and chiral aliphatic alkenes
Green Chem. 2010, 12, 815-827 comparison of bio epoxidation to chemical processes
J. Am. Chem. Soc. 2003, 125(27), 8209-8217 stereospecific biocatalytic epoxidation
Appl Environ Microbiol. 2000 May; 66(5): 1877–1882 Toluene Monooxygenase-Catalyzed Epoxidation of Alkenes
Lipase-catalyzed in situ generation of peracid
Green Chemistry 2006, 8(10), 923-926 Lipase-mediated epoxidation utilizing urea–hydrogen peroxide in ethyl acetate
J. Mol. Catal. B: Enzymatic 1995, 1(1), 29-35 Chemo-enzymatic epoxidation of unsaturated carboxylic acids
Green Chem. 2011, 13, 226−265 review – Enzyme-mediated oxidations for the chemist
Angew.Chem. Int. Ed. 2005, 44(18), 2742-2746 Molecular Basis of Perhydrolase Activity in Serine Hydrolases
Relevant scale up example
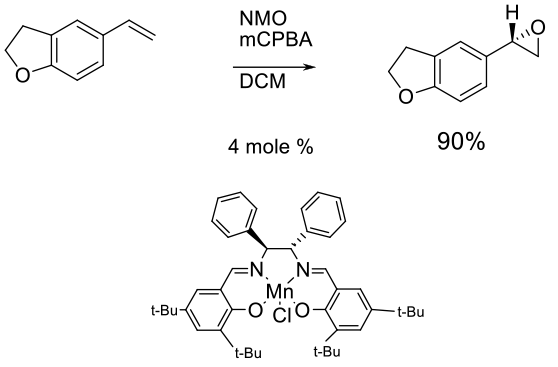
Experimental
850 gram scale
Org. Process Res. Dev. 2003, 7, 821-827
Green Review
-
Atom efficiency (by-products Mwt)
Excellent with good catalytic activity – only corresponding alcohol as by-product. Hydrolytic enzymes need no co-factors or co-factor recycling systems. - Safety Concerns
Generally biocatalytic methods are free of thermal events and can be managed in standard equipment. - Toxicity and environmental/aquatic impact
The biocatalysts are commonly biodegradable and pose minimal environmental hazards. Some of the proteins, especially dusty solids can be sensitizing (R42/H317) so appropriate handling should be employed. For use in c-GMP manufacture of API, enzymes from mammalian sources or those fermented using mammalian products should be avoided. Older work using whole cells and biphasic systems often use dialkyl phthalates as the organic phase. These materials are persistent and endocrine disruptors hence should be avoided - Cost, availability & sustainable feedstocks
Many enzymes are now becoming commercially available in bulk, and many CRO’s can offer biocatalysis and enzyme development services. In most cases, at the pilot stage, a biocatalyzed reaction will cost more than an chemical alternative (fermentation / enzyme supply is very sensitive to economies of scale), but at full scale most biocatalyzed processes are greener and cheaper than chemical alternatives. - Sustainable implications
Enzymes are produced from renewable materials and are fully biodegradable back to innocuous natural products (amino acids). The use of modern molecular biology and fermentation technology has greatly reduced the LCI of enzyme manufacture. Maximum sustainability benefits are usually obtained with mutant recombinant enzymes rather than natural enzymes.